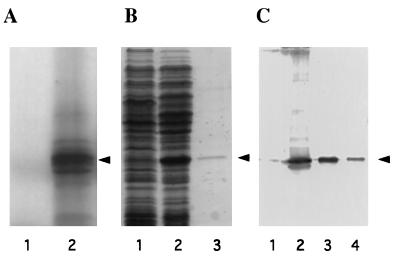
Figure 4.
Expression of the eIF6 cDNA clone. (A) SDS/15% polyacrylamide gel electrophoresis of [35S]methionine-labeled translation products in a rabbit reticulocyte transcription–translation system (TnT system, Promega). Lanes 1 and 2, in vitro translation in the presence of parental vector pBluescript SK(+) (lane 1) or recombinant vector pBluescript SK(+) containing cloned eIF6 cDNA under the control of T3 RNA polymerase promoter (lane 2). The dried gel was analyzed by autoradiography. The position of purified eIF6 is shown by an arrowhead. (B) Expression of eIF6 cDNA in E. coli. Cell-free extracts, prepared from IPTG-induced cultures of E. coli BL21 (DE3) cells harboring either the parental plasmid pET-5a (lane 1) or recombinant plasmid, pETIF6 containing the eIF6 coding region under the control of T7 RNA polymerase promoter (lane 2), and purified recombinant eIF6 (lane 3) were electrophoresed in SDS/15% polyacrylamide gel followed by Coomassie blue staining. The position of migration of eIF6 is indicated by an arrowhead. (C) Immunoblot analysis of bacterially expressed eIF6. Cell-free extracts of isopropyl β-d-thiogalactopyranoside-induced cultures of BL21 (DE3) cells harboring either the parental vector pET-5a (lane 1) or the pETIF6 recombinant expression plasmid (lane 2) were subjected to Western blot analysis by using monospecific anti-eIF6 antibodies as probes. Purified recombinant eIF6 (lane 3) and purified rabbit reticulocyte eIF6 (lane 4) were also analyzed by Western blot. A set of molecular weight marker proteins were run in a separate lane of each gel (not shown). The position of eIF6 is indicated by an arrowhead.