Fig. 1.
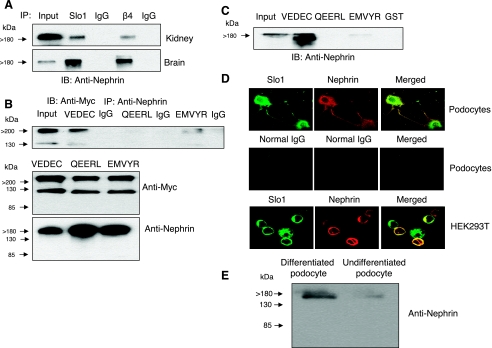
Nephrin binds to large-conductance Ca2+-activated K+ (BKCa) channel subunits. A: coimmunoprecipitation of nephrin observed by immunoblot analysis after immunoprecipitation using antibodies against Slo1 or the β4-subunit of BKCa channels from mouse kidney or mouse brain, as indicated. The “input” lane contained a diluted sample of cell extract used to illustrate electrophoretic mobility; it was not designed for quantification of signal intensity. B: coimmunoprecipitation of Slo1 observed by immunoblot analysis after immunoprecipitation with an antibody against nephrin (top). This procedure was carried out on HEK-293T cells transiently expressing Myc-tagged isoforms Slo1VEDEC, Slo1QEERL, or Slo1EMVYR, as indicated, and channels were detected using anti-Myc. Note that a strong signal was only detected from cells expressing the Slo1VEDEC isoform. Interaction with Slo1EMVYR was detectable but much weaker. IB analysis shows that the various Slo1 variants (middle) and nephrin (bottom) were expressed at comparable levels throughout the assay. C: glutathione S-transferase (GST) pull-down assay showing that a GST fusion protein containing the COOH-terminal sequences of Slo1VEDEC could bind to nephrin, whereas GST and a GST fusion protein containing the COOH-terminal sequences of Slo1QEERL did not. The interaction with Slo1EMVYR with nephrin was very weak. D: confocal microscopy showing colocalization of endogenous Slo1 and nephrin in differentiated cells of a podocyte cell line (top) and in HEK-293T cells transiently expressing nephrin and Slo1VEDEC (bottom). Middle, control images from podocytes in which the primary antibody was a species-appropriate IgG. E: immunoblot using the same commercial antibody against nephrin on extracts of differentiated and undifferentiated cells from a podocyte cell line. IB, immunoblot; IP, immunoprecipitation.