Abstract
Recent studies have implicated epithelial Na+ channels (ENaC) in myogenic signaling. The present study was undertaken to determine if ENaC and/or Na+ entry are involved in the myogenic response of the rat afferent arteriole. Myogenic responses were assessed in the in vitro hydronephrotic kidney model. ENaC expression and membrane potential responses were evaluated with afferent arterioles isolated from normal rat kidneys. Our findings do not support a role of ENaC, in that ENaC channel blockers did not reduce myogenic responses and ENaC expression could not be demonstrated in this vessel. Reducing extracellular Na+ concentration ([Na+]o; 100 mmol/l) did not attenuate myogenic responses, and amiloride had no effect on membrane potential. Benzamil, an inhibitor of ENaC that also blocks Na+/Ca2+ exchange (NCX), potentiated myogenic vasoconstriction. Benzamil and low [Na+]o elicited vasoconstriction; however, these responses were attenuated by diltiazem and were associated with significant membrane depolarization, suggesting a contribution of mechanisms other than a reduction in NCX. Na+ repletion induced a vasodilation in pressurized afferent arterioles preequilibrated in low [Na+]o, a hallmark of NCX, and this response was reduced by 10 μmol/l benzamil. The dilation was eliminated, however, by a combination of benzamil plus ouabain, suggesting an involvement of the electrogenic Na+-K+-ATPase. In concert, these findings refute the premise that ENaC plays a significant role in the rat afferent arteriole and instead suggest that reducing [Na+]o and/or Na+ entry is coupled to membrane depolarization. The mechanisms underlying these unexpected and paradoxical effects of Na+ are not resolved at the present time.
Keywords: renal autoregulation, vascular smooth muscle, sodium ion channels, sodium/calcium exchange, benzamil, amiloride, ouabain, sodium-potassium-adenosinetriphosphatase, epithelial sodium channel
the intrinsic ability of the renal afferent arteriole to elevate tone in response to increased renal perfusion pressure plays an important role in the regulation of renal function and in the protection of the kidney against hypertensive injury (26). The cellular mechanisms underlying this myogenic response are not understood. It has been reported that epithelial Na+ channels (ENaC) are expressed in the mouse interlobar artery and that activation of ENaC is involved in the vasoconstrictor response of this vessel to elevated pressure (16, 17). Previous studies had implicated ENaC in myogenic responses of cerebral vessels (7). These findings are novel in suggesting a role of inward ENaC currents in smooth muscle myogenic signaling. Although these currents had not been described in vascular myocytes, benzamil and amiloride were clearly shown to block myogenic responses in isolated rat cerebral and mouse interlobar arteries (7, 16).
The afferent arteriolar myogenic response exhibits many features that are distinct from the responses observed in larger vessels or small vessels from other vascular beds (e.g., see Ref. 23). Moreover, when considering physiological responses, it is important to note that the renal vascular sites involved in autoregulation are thought to be limited to the distal segments of the interlobular artery and the afferent arteriole. The interlobar artery is a conduit vessel. Accordingly, it is not clear to what extent findings obtained from these previous reports can be extrapolated to renal resistance arterioles. We therefore undertook to examine the effects of these Na+ transport inhibitors on the myogenic response of the afferent arteriole.
Our findings do not support the hypothesis that ENaC is involved in the myogenic response of the rat afferent arteriole in that we did not observe ENaC channel blockers to reduce myogenic responses in this vessel and we could not demonstrate intact ENaC expression in isolated afferent arterioles. Moreover, benzamil, which is also an inhibitor of Na+/Ca2+ exchange (NCX) (2, 20), actually potentiated myogenic vasoconstriction. This latter observation prompted further studies examining the effects of alterations in the Na+ gradient on the afferent arteriole. Although the results of these studies do provide support for previous reports (1, 10, 30, 33, 34) suggesting an important role of NCX in modulating afferent arteriolar tone, we found unanticipated effects of benzamil and altered extracellular Na+ concentration ([Na+]o) on the membrane potential that cannot be explained by such a mechanism. These findings confound simple interpretations of studies employing these approaches to investigate the role of NCX in this vessel.
METHODS
In vitro perfused hydronephrotic rat kidney.
The in vitro perfused hydronephrotic kidney model was used to assess the effects of Na+ transport inhibitors and altered [Na+]o on the renal afferent arteriole. This preparation is described in detail in previous publications (e.g., see Refs. 19, 23, and 37). In brief, chronic unilateral hydronephrosis is induced by ligating the left ureter of male Sprague-Dawley rats (80–100 g). Complete atrophy of the tubular elements is seen after 6–8 wk, allowing direct visualization of the renal microvasculature. At this time, the hydronephrotic kidney is harvested for in vitro perfusion. For harvesting, the left renal artery is cannulated in situ, and the kidney is excised and transferred to a heated chamber on the stage of an inverted microscope. This entire procedure is accomplished without disrupting perfusion of the kidney. The protocol for this study was approved by the University of Calgary Animal Care Committee, and the procedures comply with the regulations of the Canadian Council on Animal Care.
Hydronephrotic kidneys were perfused with a modified DMEM containing 30 mmol/l bicarbonate, 5 mmol/l glucose, and 5 mmol/l HEPES (pH 7.40) and equilibrated with 5% CO2-95% air. Temperature was maintained at 37°C. Renal arterial pressure (perfusion pressure) was measured within the renal artery. Medium was pumped on demand through a heat exchanger to a pressurized reservoir feeding the renal arterial cannula. All drugs were added directly to the perfusate. The venous effluent drains into the bath, providing access to both abluminal and adluminal surfaces of the vessels. A fiber optic probe was positioned to stabilize and transilluminate a portion of the membranous renal cortex for observations of renal microvascular responses. Afferent arteriolar diameters were measured by on-line image processing. Diameter measurements were obtained at each pixel and averaged over the entire segment length (20–40 μm). Data were collected at a scanning rate of 5–8 Hz, and mean diameter measurements were averaged over the plateau of each response.
Kidneys were allowed to equilibrate for at least 1 h following the establishment of in vitro perfusion. Ibuprofen (10 μmol/l) was added to eliminate the effects of endogenous prostaglandins (37). Basal renal perfusion pressure was held at 80 mmHg during the equilibration period. In some experiments, myogenic responses were evoked by raising renal arterial pressure and holding pressures at each step for at least 1 min. In these studies, stepped responses were assessed before and after the administration of amiloride or benzamil. In other experiments, perfusion pressure was maintained at a constant level (60, 80, or 140 mmHg) to assess vasoconstrictor and/or vasodilatory responses. In the studies assessing the impact of low [Na+]o media, the media Na+ concentration ([Na+]) was lowered from 140 to 100 mmol/l by the isosmotic substitution of choline chloride for NaCl. Benzamil, amiloride, and ouabain were obtained from Sigma (St. Louis, MO). Fresh stock solutions of benzamil and amiloride were prepared in dimethyl sulfoxide. Ouabain (3 mmol/l in DMEM) was prepared fresh for each experiment. Phentolamine and propranolol (10 μmol/l; Sigma) were added during the ouabain experiments to avoid any effects mediated by neurotransmitter release.
Isolated afferent arterioles.
Afferent arterioles were isolated from normal rat kidneys using the gel-perfusion technique developed in our laboratory (27). In brief, rats were anesthetized, and the left kidney was perfused in vivo with warmed modified minimal essential medium (MEM). The kidney was perfused with 37°C agarose (seaprep, 1.5%), excised, and chilled (4°C) to solidify the agarose. Cortical slices (400 μm) were incubated in Ca2+-free MEM containing 5 mM HEPES, 3.9 mM NaHCO3, and atmospheric CO2. Slices were treated with collagenase IV, dispase II, and DNase I to separate microvessels from tubules. Individual vessels were isolated using a dual pipette system (27). For RT-PCR studies, arterioles were washed by transferring to a clean dish two times before the final isolation. For positive controls, single bifurcating segments of the cortical collecting duct (CCD) were collected, and intact segments or freshly dispersed (principal) CCD cells were processed for RT-PCR using the same assay. As additional controls, intact interlobar arteries and myocytes freshly obtained from an interlobar artery were isolated and processed as described above.
For membrane potential measurements, single afferent arterioles were placed in a heated (36°C) chamber on the stage of an inverted microscope and superfused with MEM containing normal bicarbonate and 5% CO2. Borosilicate glass pipettes, pulled to a 2- to 3-μm tip diameter, were briefly back-dipped in solution containing (in mmol/l) 120 potassium gluconate, 20 KCl, 10 HEPES, 5 EGTA, 2 MgCl2, and 0.1 CaCl2 (pH 7.2) to fill the pipette tip, and then the remainder of the pipette was backfilled with the same solution containing 100 μg/ml nystatin. A seal was obtained on a myocyte of the afferent arteriole, and electrical access via nystatin was confirmed by a marked decrease in the access resistance coupled with the development of a stable membrane potential, typically on the order of −60 mV. Membrane potential recordings were obtained in current clamp mode (current = 0). Data were acquired at 10 Hz and filtered at 1 kHz. To derive the “true” membrane potential using this approach, it is necessary to correct the measured potential for the zeroing of the pipette-bath liquid junction potential before establishing the membrane seal (29) and also for a Donnan potential set up across the perforated patch that is dependent on the intracellular concentration of nondiffusible anions (31). Measurements of the junction potential as described (29) indicated that it was ∼8 mV (bath − pipette potential). The second error was calculated as ∼4 mV (pipette − cell potential), assuming an intracellular nondiffusible anion concentration of 100 mM (31). This implied that the actual membrane potential was ∼4 mV more negative than the recorded potential. However, because we were interested in changes in membrane potential, rather than its absolute value, the observed voltages were not corrected.
For RT-PCR studies, total RNA was prepared from pooled samples of 2–10 afferent arterioles and from the controls described above using the RNeasy Micro kit (QIAGEN) and eluted in 12 μl RNase-free water. cDNA was prepared from the entire eluate using the Sensiscript RT kit (QIAGEN) combined with 9 μmol/l random nonamers and 1 μmol/l oligo(dT) (37°C, 1 h). ENaC subunit (α, β, and γ) and β-actin expression were analyzed using a nested RT-PCR approach. The outer and inner primers are shown in Table 1. Note that two different primer sets were used to assay for the γ-subunit of ENaC (see Table 1). PCR was performed with a HotStarTaq Master Mix kit (first reaction) and a Taq PCR Master Mix kit (second reaction; QIAGEN). Following the initial amplification (1 cycle at 95°C for 15 min and 35 cycles at 94°C for 30 s, 56°C for 30 s, and 72°C for 60 s, followed by 72°C for 10 min), the product was diluted 200-fold and subjected to the second round PCR using the Taq PCR Master Mix kit (1 cycle at 94°C for 3 min and 35 cycles at 94°C for 30 s, 56°C for 30 s, and 72°C for 60 s, followed by 72°C for 10 min). Products were separated by gel electrophoresis and visualized by ethidium bromide. The identity of the products was confirmed by sequencing (University of Calgary Sequencing Facility; www.sequencing.ucalgary.ca).
Table 1.
Primers used for RT-PCR assays
| Forward Primers | Reverse Primers | Product Size, bp | Ref. Sequence | |
|---|---|---|---|---|
| ENaC-α | ||||
| 1st | TCCCAGGATTGGATCTTCGAG | ACCACAGAGAGCACGGACGA | 209 | |
| 2nd | GAGATGCTGTCCTTGCAGAAC | GGACGAGCCAAACCACAGG | 177 | NM031548 |
| ENaC-β | ||||
| 1st | GACCATCTCCATGGCTGACTG | GGTTAGAGAGCAGCCACACG | 200 | |
| 2nd | CGAGGCCTCTGAGGATTG | CAGCCACACGATATTGTTGG | 163 | NM012648 |
| ENaC-γ1 | ||||
| 1st | TCTGAGGCTTCCGAGAAATG | CCACCAAAGTTGGACAGGAG | 182 | |
| 2nd | ATGGTTGCTGAATGTTCTCAC | CCACCAAAGTTGGACAGGAG | 165 | NM017046 |
| ENaC-γ2 | ||||
| 1st | CCCTCCTCGTCTTCTCTTTCT | CCCATACAAGGACAGCAAGG | 176 | |
| 2nd | CCGTCTCTGTCTCCATCAAAG | GCCTGTTTTGTCTCACTGTCC | 133 | NM017046 |
| β-Actin | ||||
| 1st | CTCATGAAGATCCTGACCGAG | CTAGGAGCCAGGGCAGTAAT | 404 | |
| 2nd | CTGTGCTATGTTGCCCTAGACTTC | CATCCTGTCAGCAATGCCTG | 294 | NM031144 |
ENaC, epithelial Na+ channel.
All data are presented as means followed by the SE as an index of dispersion. Differences between treatment groups were assessed by one-way analysis of variance and the Bonferroni test for multiple comparisons. Differences between means exhibiting P values <0.05 were considered to be statistically significant.
RESULTS
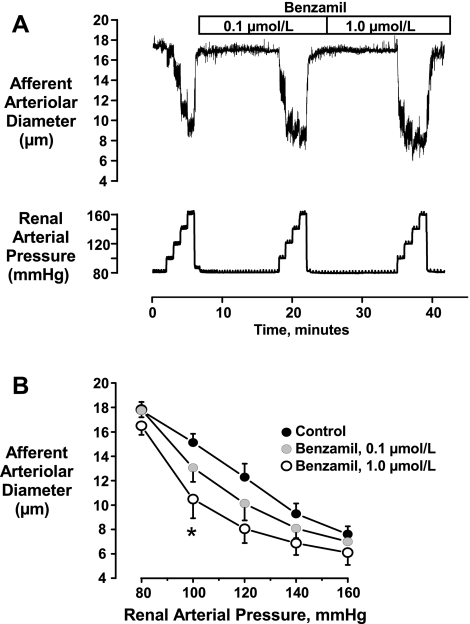
The tracing shown in Fig. 1A shows the protocol used to examine the effects of ENaC blockade on the afferent arteriolar myogenic response. As shown by the mean data presented in Fig. 1B, low concentrations of benzamil (0.1–1.0 μmol/l) had no effect on the basal diameters observed at 80 mmHg. At 0.1 μmol/l, benzamil had no significant effect on myogenic responses at any pressure (P > 0.5). However, at 1.0 μmol/l, benzamil caused a significantly greater myogenic vasoconstriction, which attained statistical significance at 100 mmHg (15.1 ± 0.7 vs. 10.5 ± 1.6 μm, P < 0.05, n = 6).
Fig. 1.
Original tracing (A) and mean data (B, n = 6) showing the effects of benzamil on myogenic responses of the afferent arteriole. Note that rather than blocking myogenic reactivity, 1.0 μmol/l benzamil actually enhanced the vasoconstriction (*P < 0.05 vs. control response).
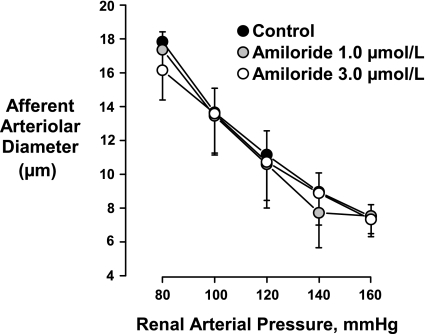
As shown in Fig. 2, amiloride had no significant effect on the myogenic response at concentrations of 1 and 3 μmol/l (P > 0.05 for all pressures, n = 5). Similar results were obtained in the absence of ibuprofen (data not shown). Because, at the concentrations employed, amiloride and benzamil block ENaC (2, 7), the results of these experiments, using the hydronephrotic kidney model, do not support the postulate that this Na+ channel plays a prominent role in myogenic signaling in the rat afferent arteriole. To further evaluate this postulate, we determined if afferent arterioles isolated from normal rat kidneys expressed ENaC subunits.
Fig. 2.
Amiloride had no significant effect on the myogenic response of the afferent arteriole to changes in renal arterial pressure (n = 5).
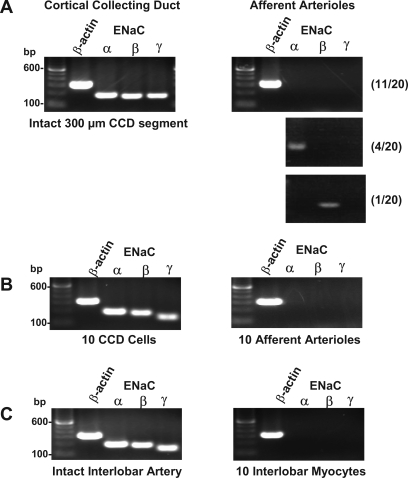
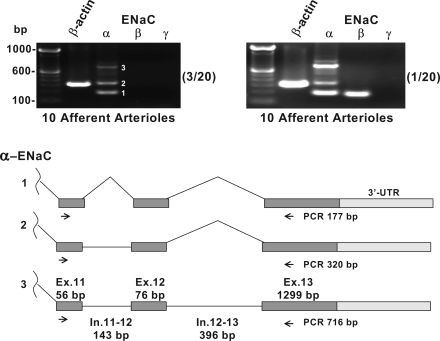
The results of the RT-PCR studies are depicted in Figs. 3 and 4. Afferent arterioles were isolated from 20 separate kidney preparations. Two different ENaC-γ primer sets were developed. Fig. 3A depicts representative RT-PCR results using the α, β and γ1 primers (described in Table 1). As shown, this assay was sensitive enough to detect strong ENaC mRNA signals for all ENaC subunits in a small 300-μm segment of the CCD. Figures 3B, 3C, and 4 depict representative findings using the same α and β primers and the γ2 primer set. This assay was sensitive enough to detect strong signals for all ENaC subunits in only 10 CCD epithelial cells. We saw no evidence of afferent arteriolar expression of the ENaC γ-subunit in any of the 20 determinations. In 11 of the 20 assays, we found no evidence of afferent arteriolar expression of any of the three ENaC subunits. In four assays, a single PCR product was found that corresponded in size and sequence to the predicted ENaC α-subunit. In 2 of the 20 assays, we found evidence for the presence of the ENaC β-subunit (Figs. 3A and 4). Interestingly, we found three separate PCR products from the ENaC α-subunit primers in 4 of the 20 assays (Fig. 4, top). These primers were designed to span two introns and three exons (Fig. 4, bottom). The three PCR products were sequenced and found to correspond to the unprocessed, partially processed, and fully processed mRNA shown in this diagram. Omission of reverse transcriptase treatment (−RT) eliminated all three bands (data not shown). Considering that these precursor mRNAs are likely to be present in very low abundance, the detection of these signals illustrates the high sensitivity of the nested primer assay. Finally, it was requested that we use a vascular preparation as a second positive control. As shown in Fig. 3C, we detected all three ENaC subunits in a single segment of the intact interlobar artery. We noted that this conduit vessel has a complex adventitial layer that we could not fully remove. We therefore attempted to detect expression in myocytes isolated from this vessel. Unlike the intact vessel, the rat interlobar myocytes did not express ENaC message at levels that could be detected using our assay. It should be noted that ENaC α-, β-, and γ-subunit mRNAs have been detected in cultured interlobar myocytes obtained from the mouse (16).
Fig. 3.
A: representative RT-PCR assays of afferent arterioles using the α, β, and γ1 nested primers (Table 1, 35 + 35 cycles) and single (300 μm) segment of the cortical collecting duct (CCD). Nos. in parentheses indicate frequency of observation in all 20 kidney preparations. B: with the use of the α, β, and γ2 primers (Table 1), mRNA for all three epithelial Na+ channel (ENaC) subunits could be detected in 10 CCD epithelial cells but not in 10 afferent arterioles (∼1,000 cells). C: same assay could detect ENaC subunit expression in an intact interlobar artery but not in isolated interlobar myocytes (see text for description).
Fig. 4.
As shown in top, the ENaC α-subunit assay produced multiple PCR products in 4/20 assays (labeled 1–3 in top left). These three products were sequenced and found to correspond to the predicted processed, partially processed, and unprocessed mRNA sequences shown in bottom. These products were not seen in −RT control (data not shown). Nos. in parentheses represent the frequency of these observations in the 20 RT-PCR assays.
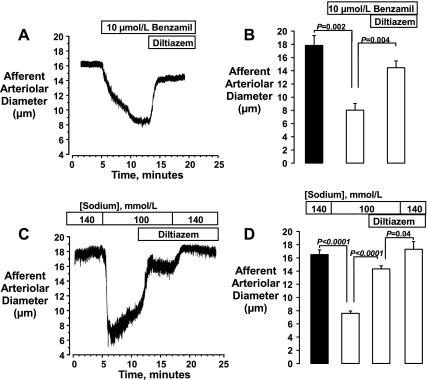
The potentiating effects of 1.0 μmol/l benzamil on the vasoconstrictor response of the afferent arteriole to pressure was unexpected with regard to its characterization as an ENaC inhibitor but would be consistent with the ability of benzamil to block the forward mode of NCX (2). We set out to evaluate this possibility by examining the effects of benzamil on Na+-induced vasodilator responses attributed to NCX. In preparation for these studies, we first examined the effects of a higher concentration of benzamil (10 μmol/l) and of reducing [Na+]o on the afferent arteriole. As shown in Fig. 5, both manipulations resulted in marked afferent arteriolar vasoconstriction. At normal [Na+]o (140 mmol/l), benzamil reduced afferent arteriolar diameters from 17.9 ± 1.5 to 8.0 ± 1.0 μm (P = 0.002, n = 4). The isosmotic reduction of [Na+]o from 140 to 100 mmol/l reduced diameters from 16.5 ± 0.7 to 7.6 ± 0.4 μm (P < 0.0001, n = 5). Diltiazem substantially reversed each of these vasoconstrictor responses, returning diameters to 14.5 ± 1.0 μm (P = 0.004 vs. benzamil alone) and to 14.3 ± 0.5 μm (P < 0.0001 vs. low [Na+]o alone). In the group of kidneys equilibrated with low [Na+]o, the restoration of normal [Na+]o caused a further vasodilation in the presence of diltiazem (from 14.3 ± 0.5 to 17.3 ± 1.2 μm, P = 0.04).
Fig. 5.
Original tracings (left) and mean data showing effects of 10 μmol/l benzamil (A and B, n = 4) and reducing extracellular Na+ concentration ([Na+]o) from 140 to 100 mmol/l (C and D, n = 5) on the afferent arteriole. Kidneys were perfused at an arterial pressure of 80 mmHg. Benzamil and reduced [Na+]o each elicited a marked vasoconstriction that was partially reversed by diltiazem (10 μmol/l). Note that, in regard to the latter, restoration of normal [Na+]o reversed the diltiazem-insensitive component of this response (C and D).
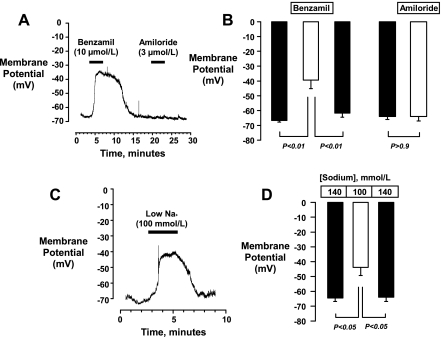
The vasoconstriction elicited by low [Na+]o and by benzamil and the observation that both responses were reversed by diltiazem suggest that these manipulations might evoke membrane depolarization. To investigate this possibility, we measured membrane potential responses in afferent arterioles isolated from normal kidneys using current clamp techniques employing the perforated patch approach. Examples of voltage tracings are presented in Fig. 6, A and C. Benzamil (10 μmol/l) evoked an ∼27-mV depolarization (−67 ± 1 to −40 ± 6 mV, P < 0.01, n = 4, Fig. 6B) that was reversed upon removal of benzamil from the bath (−62 ± 3 mV, P < 0.05 vs. benzamil). In contrast to benzamil, amiloride (3 μmol/l) had no effect on membrane potential (−64 ± 2 vs. −64 ± 3 mV, n = 3, Fig. 6, A and B). Low [Na+]o (100 mmol/l) evoked a response similar to that of benzamil, depolarizing the membrane potential from −65 ± 2 to −44 ± 6 mV (P < 0.05, n = 4, Fig. 6, C and D). Membrane potential returned to −64 ± 3 mV (P > 0.05 vs. control) when normal [Na+] was restored.
Fig. 6.
Original tracings (left) and mean data showing effects of 10 μmol/l benzamil (n = 4), 3 μmol/l amiloride (A and B, n = 3), and reducing [Na+]o from 140 to 100 mmol/l (C and D, n = 4) on the membrane potentials of afferent arterioles isolated from normal rat kidneys. Benzamil and low [Na+]o caused a significant and reversible membrane depolarization. Amiloride had no effect.
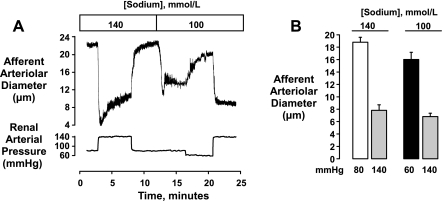
We next determined whether myogenic vasoconstriction could be elicited in low [Na+]o. The protocol for these studies is shown by Fig. 7A. In six of the nine studies in this series, we first examined the response to elevating renal arterial pressure to 140 mmHg in normal [Na+]o and then determined the response in low [Na+]o. In the total group, reducing [Na+]o resulted in an initial afferent arteriolar vasoconstriction from 17.2 ± 0.9 to 9.7 ± 0.9 μm (P <0.0001, n = 9). Reducing renal arterial pressure from 80 to 60 mmHg dilated the vessels to 14.6 ± 1.1 μm. Previous work had shown that reducing perfusion pressure in this preparation elicits a hyperpolarization [−40 ± 1 mV at 80 mmHg (24) vs. −47 ± 2 mV at 40 mmHg (5)]. Elevation of renal arterial pressure from 60 to 140 mmHg in the continued presence of reduced [Na+]o (100 mmol/l) resulted in a myogenic vasoconstriction to 6.2 ± 0.5 μm (Fig. 7B), a level similar to that attained in 140 mmol/l Na+ (7.8 ± 0.9 μm, P = 0.10, n = 6).
Fig. 7.
Myogenic responses in normal (140 mmol/l) and low [Na+]o (100 mmol/l). Note that following the low [Na+]o-induced vasoconstriction, diameters could be partially restored by reducing perfusion pressure from 80 to 60 mmHg (tracing A, mean data, B). Myogenic responses to elevating renal arterial pressure to 140 mmHg could then be elicited (n = 9), and the level of myogenic tone attained was similar to that elicited in normal [Na+]o (n = 6). Statistical analyses are described in the text.
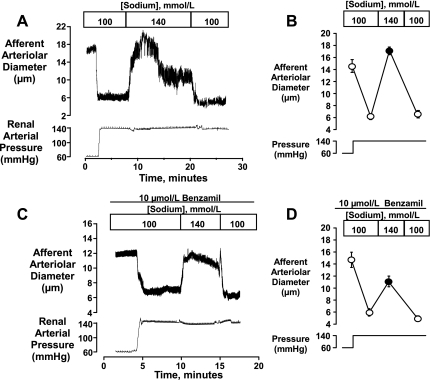
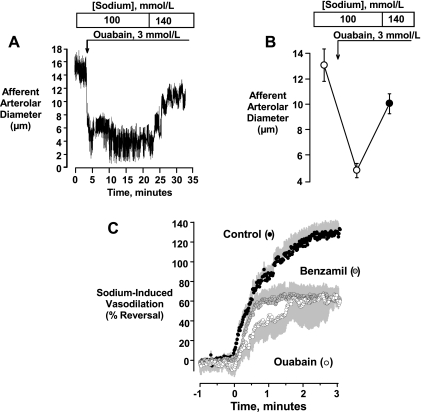
Because reducing [Na+]o did not attenuate pressure-induced afferent arteriolar vasoconstriction, we used this approach to study NCX in this preparation and evaluated the vasodilatory response evoked by restoring [Na+]o to normal levels. An original tracing and mean data are depicted in Fig. 8, A and B. In this series, elevating renal arterial pressure from 60 to 140 mmHg elicited afferent arteriolar vasoconstriction from 14.6 ± 1.1 to 6.2 ± 0.5 μm (P < 0.001, n = 9) in the presence of 100 mmol/l [Na+]o. When [Na+]o was increased to 140 mmol/l, a rapid and complete vasodilation was elicited that returned diameters to 17.1 ± 0.6 μm (P < 0.001). This initial vasodilation persisted for at least 10 min and then partially abated. When [Na+]o was again reduced to 100 mmol/l, diameters returned to the initial level of vasoconstriction (6.6 ± 0.6 μm). In a subset of six kidneys, the initial and sustained components of the response were quantified. Arteriolar diameters were 16.0 ± 1.2 and 6.8 ± 0.5 μm at 60 and 140 mmHg, respectively, and initially increased to 17.8 ± 0.8 μm upon raising [Na+]o to 140 mmol/l. This peak response persisted for ∼10 min, after which diameters returned to 12.4 ± 1.0 μm (P < 0.001 vs. initial peak response), a level of vasodilation that remained significant (P < 0.001 vs. 100 mmol/l Na+).
Fig. 8.
Na+-induced vasodilation seen when [Na+]o was increased from 100 to 140 mmol/l. Afferent arterioles were preconstricted by elevating renal perfusion pressure from 60 to 140 mmHg. As shown in A and B, restoration of normal [Na+]o resulted in an initial vasodilation that restored diameters to values greater than that seen at 60 mmHg (n = 9). The initial vasoconstriction was restored upon returning [Na+]o to 100 mmol/l. C and D show that partially attenuated vasodilatory responses could be elicited in the presence of 10 μmol/l benzamil (n = 6). Statistical analyses are described in the text.
The vasodilator response that was observed when normal [Na+]o was restored, hereafter referred to as the Na+-induced vasodilation, was only partially attenuated by 10 μmol/l benzamil. These data are depicted in Fig. 8, C and D. In this series (n = 6), control diameters were 18.0 ± 1.5 μm at a perfusion pressure of 80 mmHg. Following equilibration with 10 μmol/l benzamil and low [Na+]o (100 mol/l) at a perfusion pressure of 60 mmHg, diameters were 14.7 ± 1.3 μm. Elevating perfusion pressure to 140 mmHg resulted in a vasoconstriction to 6.0 ± 0.6 μm (P < 0.001). Increasing [Na+]o caused a peak vasodilation to 11.1 ± 0.9 μm (P = 0.01 vs. 100 mmol/l [Na+]o). Diameters returned to 5.0 ± 0.5 μm when [Na+]o was again reduced to 100 mmol/l. As shown in Fig. 9, A and B, a Na+-induced vasodilation could also be elicited when vasoconstriction was elicited by ouabain, rather than by elevated pressure. As previously shown in our laboratory (e.g., 6), blockade of the Na+-K+ ATPase with 3 mmol/l ouabain elicits a marked vasoconstriction of the rat afferent arteriole. In this series (n = 5), control diameters were 19.0 ± 0.7 μm at 80 mmHg. Following equilibration in low [Na+]o media (100 mmol/l) at 60 mmHg, diameters were 13.1 ± 1.3 μm. The administration of 3 mmol/l ouabain reduced diameters to 4.8 ± 0.6 μm (P < 0.001). In this setting, elevating [Na+]o to 140 mmol/l elicited a more slowly developing vasodilation, returning afferent arteriolar diameters to 10.0 ± 0.8 μm (P < 0.01 vs. ouabain alone, P > 0.05 vs. preouabain). Figure 9C compares the time courses for the Na+-induced vasodilation seen in controls, and with benzamil or ouabain treatment. Note the slower time course seen in the presence of ouabain and the apparent presence of both a fast and slow component in the controls.
Fig. 9.
Na+-induced vasodilation could also be elicited in the presence of ouabain. Ouabain (3 mmol/l) produced a marked vasoconstriction of the afferent arteriole (renal arterial pressure held constant at 60 mmHg) in the low [Na+]o media. Restoring [Na+]o to 140 mmol/l resulted in a more slowly developing vasodilation. Mean data (n = 5) shown in B. Statistical analysis is described in the text. Time courses for the vasodilatory responses in controls, benzamil, and ouabain are compared in C.
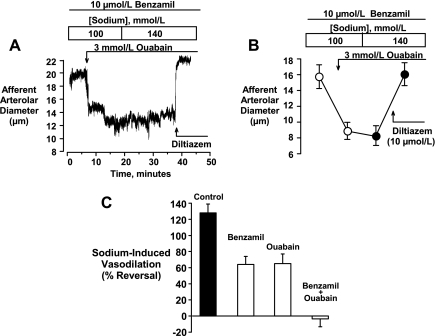
Because a partially attenuated Na+-induced vasodilation could be elicited in the presence of either benzamil or ouabain, we next determined if the combination of these two agents might fully eliminate this response. In these experiments, shown in Fig. 10, A and B, basal diameters in 100 mmol/l [Na+]o at 60 mmHg were 15.7 ± 1.5 μm (n = 6). Ouabain (3 mmol/l) constricted the vessel to a diameter of 8.9 ± 1.1 μm (P < 0.01) and increasing [Na+]o to 140 mmol/l had no significant effect (diameters 8.3 ± 1.3 μm, P > 0.05 vs. 100 mmol/l Na+). A full vasodilation could, however, be elicited by the application of 10 μmol/l diltiazem (16.1 ± 1.4 μm, P > 0.05, vs. control). Figure 10C summarizes the effects of benzamil and ouabain on the dilation induced by Na+, expressing the control response and the responses in the presence of these two agents as the percent reversal of vasoconstriction.
Fig. 10.
Original tracing (A) and mean data (B, n = 6) illustrating that the combined treatment with benzamil (10 μmol/l) and ouabain (3 mmol/l) completely eliminated Na+-induced vasodilation. Note that diltiazem (10 μmol/l) was able to fully reverse the vasoconstriction while Na+ replacement had no effect. Statistical analyses are described in the text. C compares the magnitude of responses observed in each setting.
DISCUSSION
Our interpretation of the data obtained in this study is that ENaC is not involved in signaling events underlying the myogenic response of the rat renal afferent arteriole. As discussed below, this conclusion is based on observations obtained using a number of different approaches. One involved reducing the driving force for Na+ entry by lowering [Na+]o. This manipulation promoted afferent arteriolar vasoconstriction, which was reversed by Na+ repletion. One mechanism contributing to this effect may be a reduction in Ca2+ extrusion via NCX. A second mechanism may involve the effects of reducing Na+ entry on the activity of the electrogenic Na+-K+-ATPase, since ouabain was found to attenuate a rapid component of the vasodilatory response to Na+. We observed, however, that both low Na+ and the ENaC/NCX blocker benzamil evoked afferent arteriolar membrane depolarization. The mechanism(s) causing these depolarizations are presently unclear. Potential mechanisms might include the evocation of a Ca2+-activated Cl− current secondary to rises in intracellular Ca2+ concentration caused by NCX inhibition (e.g., 34). In any case, the depolarization elicited by these manipulations complicates the interpretations of our experiments addressing the influence of NCX on this vessel and also highlights the dangers inherent in viewing the effects of benzamil purely in terms of ENaC and/or NCX blockade.
It has previously been reported that myogenic responses of small cerebral arteries of the rat and of the mouse interlobular artery are inhibited by blockade of ENaC using 0.1–1.0 μmol/l benzamil and 1–5 μmol/l amiloride (7, 16, 17). More recently, the same laboratory found that the myogenic responses of the rat mesenteric resistance arteries are not attenuated by benzamil, unless animals were placed on a high-salt diet (18). The effects of amiloride on the mesenteric myogenic response were not reported in this latter study. In the present study, we found amiloride to have no effect on the myogenic response of the afferent arteriole and observed that benzamil not only failed to inhibit the response but actually potentiated pressure-induced vasoconstriction. Similarly, reducing the inward Na+ gradient had no significant effect on the myogenic response in that similar vasoconstrictor responses were observed at 140 mmHg in the presence of 100 and 140 mmol/l [Na+]o (Fig. 7). Reducing [Na+]o not only failed to impair myogenic reactivity, but, as discussed below, this manipulation elicited afferent arteriolar depolarization and vasoconstriction. Taken together, these findings indicate that ENaC is not involved in myogenic signaling in the renal afferent arteriole as assessed in the hydronephrotic rat kidney preparation. We point out that the myogenic response of this model has been characterized extensively. Previous findings demonstrate not only that the effects of physiological, pathophysiological, and pharmacological interventions correspond to the effects on autoregulatory responses of the intact rat kidney (12–14, 19, 25, 36, 38) but also that the kinetic attributes of the myogenic response of this preparation are identical to those of the intact kidney of the conscious rat (6, 23). Given the close concordance between these observations, it is most likely that the mechanisms underlying the myogenic response of this preparation correspond to that of the normal afferent arteriole.
Despite these similarities, it was suggested that the mechanisms underlying the myogenic response in the hydronephrotic kidney model might differ from those of the normal kidney, since it was recently reported by Li et al. (22) that ENaC expression in the CCD is rapidly downregulated following ureteral obstruction. This same group (12) had previously surveyed ENaC expression in the rat kidney. They did not report ENaC expression in the renal vasculature nor did they find vascular effects of obstruction in the more recent study (Dr. J. Frökiaer and Dr. S. Nielsen, personal communication). Nevertheless, to address the specific concern that ENaC expression might be reduced in the hydronephrotic kidney model and that this might lead to the lack of effect of ENaC blockers, we assayed expression levels in afferent arterioles isolated from normal rat kidneys. ENaC is a heteromeric construct comprised of α-, β-, and γ-subunits [proposed stoichiometry of 2:1:1 (11)]. mRNAs for all three subunits are reported to be expressed in intact isolated cerebral arteries (7), in cultured smooth muscle cells from mesenteric resistance vessels (16), and in cultured endothelial cells (21). Cerebral myocytes express the β- and γ-subunits of ENaC but not the α-subunit (7). In the present study, we conducted an extensive survey using 20 separate afferent arteriolar preparations. The nested RT-PCR assay employed 35 + 35 cycles and was sensitive enough to detect mRNA for all three ENaC subunits in just 10 CCD epithelial cells (Fig. 3B). In 11 of the 20 assays, we could not detect any ENaC subunit mRNA (Fig. 3A). Four assays detected the predicted α-subunit product (Fig. 3A). An additional four assays detected larger mRNAs that, on analysis, were found to include incompletely modified α-subunit mRNA transcripts (Fig. 4). The latter also validates the sensitivity of the assay, since the unmodified transcripts would likely be present at very low levels. Two of the 20 assays detected mRNA for the ENaC β-subunit. The ENaC γ-subunit was not detected in any of the 20 assays, despite the fact that two primer sets were used. Accordingly, we found no evidence for intact ENaC channel expression (α-, β-, and γ-subunits) or for the expression of the ENaC β-γ construct in the renal afferent arteriole. We cannot distinguish whether the detection of the α- and β-subunits represents a contamination from epithelial cell mRNA encountered during the isolation procedure or true expression. The detection of unprocessed α-subunit mRNA may suggest the latter, although, in this case, the level of expression would appear to be quite low. The reader is cautioned that ENaC mRNA, of course, may be present but below the level of detection by our assay and that the lack of detectable levels of ENaC mRNA does not necessarily mean that the ENaC proteins are lacking. Our observation that amiloride had no effect on afferent arteriolar membrane potential, however, suggests that, if ENaC is expressed at all in this vessel, the density of the expressed channel is insufficient to provide a role in the control of membrane potential.
Benzamil treatment not only failed to inhibit the myogenic response but significantly potentiated the vasoconstriction (Fig. 1). Although a potent inhibitor of ENaC, benzamil is known to affect other Na+ transporters, including NCX (20). A number of previous studies have demonstrated an importance of NCX in regulating the renal microvasculature (1, 10, 30, 33, 34). Fowler et al. (10) and Nelson et al. (30) demonstrated, by Ca2+ signaling, that NCX activity is particularly prominent in the afferent arteriole. Studies in the isolated perfused rat kidney model by Schweda et al. (33, 34) also indicate an important role of NCX in regulating renal vascular resistance. In support of these previous observations, we found that Na+ restoration elicited afferent arteriolar vasodilation in pressurized vessels preequilibrated in low [Na+]o and that this vasodilation was attenuated by 10 μmol/l benzamil. A partial inhibition of NCX by 1.0 μmol/l benzamil could thus contribute to its potentiating effects on the myogenic response. Amiloride, which is ∼10-fold less potent against NCX (2, 20), showed no such effect. Although this interpretation is consistent with the current view in regard to the potential importance of NCX in the afferent arteriole, some aspects of our observed effects of benzamil and altered Na+ suggest that more complex mechanisms are involved.
We found that benzamil caused afferent arteriolar membrane depolarization, an effect that cannot be explained by its actions on either ENaC or NCX and may have contributed to the potentiation of vasoconstriction. Similarly, reducing [Na+]o also resulted in a depolarization that was reversed upon [Na+]o restoration. These membrane potential effects of benzamil and [Na+]o confound the interpretations of these studies examining the potential role of NCX. Finally, we found that ouabain inhibited a rapid component of the Na+-induced vasodilation, suggesting that alterations in [Na+]o may impact on the activity of the electrogenic Na+-K+-ATPase and influence afferent arteriolar contractility via effects on membrane potential that are independent of NCX.
Our observations that reducing [Na+]o and the administration of benzamil both elicited membrane depolarization in isolated afferent arterioles are novel, and these findings are consistent with the diltiazem-sensitive afferent arteriolar vasoconstriction we observed in the hydronephrotic kidney preparation. These findings are also consistent with the previous reports of Schweda and coworkers (33, 34). These investigators reported that low [Na+]o, benzamil, and KB-R7943 elicited vasoconstriction in the isolated perfused rat kidney and that these responses were reversed by amlodipine (33, 34). Although NCX is electrogenic, this transporter generates an inward, depolarizing current when operating in the forward mode (3 Na+ exchanged for each Ca2+ extruded). Thus a reduction in NCX current would tend to hyperpolarize, not depolarize. Similarly, a reduction in the inward current carried by ENaC would result in hyperpolarization, and amiloride had no effect on membrane potential. Accordingly, the observed membrane depolarization cannot be due to effects on either NCX or ENaC. As shown in Fig. 5, C and D, a diltiazem-insensitive component of the low [Na+]o-induced vasoconstriction was also evident, and the effects of reducing Ca2+ extrusion via NCX may directly contribute to this component of the response.
The mechanisms underlying the effects of altering [Na+]o on the membrane potential of the afferent arteriole are not resolved. We could not find any reports in the literature describing similar effects of [Na+]o on other blood vessel types or other smooth muscle preparations. It is interesting in this regard that this effect is so prominent in the afferent arteriole. An understanding of this phenomenon might lead to insights into the unique determinants of reactivity of this important vessel. In transporting epithelium, alterations in the Na+ gradient alter Na+ entry and affect the activity of the electrogenic Na+-K+-ATPase (3); however, to our knowledge, this mechanism has not been previously suggested to play a regulatory role in vascular smooth muscle. The finding that ouabain appears to block a rapid component of the vasodilation evoked by elevated [Na+]o (Fig. 9) would be consistent with such a mechanism, and Na+-K+-ATPase is reported to be sensitive to physiological changes in intracellular [Na+] (9, 35). The outward current attributed to the operation of the ouabain-sensitive Na+-K+-ATPase, however, is generally quite small [∼1 pA/pF, guinea pig mesenteric artery myocyte (28)], and estimates concerning its contribution to the smooth muscle membrane potential vary considerably (e.g., 4, 28, 32, 39). This issue has not been adequately addressed in the afferent arteriole. The mechanisms underlying the membrane potential effects of benzamil on the afferent arteriole will also require further investigations. Benzamil affects a number of Na+ transporters (8, 20) and may also impact on Na+ entry. Amiloride, which shares some of these characteristics, did not exhibit this effect on membrane potential. Schweda et al. (34) suggested that benzamil and KB-R7943 might elicit membrane depolarization by opening Ca2+-activated Cl− channels secondary to inhibition of NCX. Further studies are required to address this issue.
In conclusion, we interpret the findings of the present study to indicate that ENaC is not involved in myogenic signaling in the rat afferent arteriole and that intact ENaC mRNA is not expressed in this vessel. Extracellular Na+ and/or Na+ entry does, however, have an important influence on the reactivity of the afferent arteriole. This effect is not related to ENaC, since reducing [Na+]o elicited vasoconstriction. Although a depression of NCX may contribute to this response, membrane depolarization and activation of L-type Ca2+ channels appear to play more prominent roles. The mechanisms underlying the effect of altered [Na+] on the membrane potential of the afferent arteriole remain to be defined.
GRANTS
These studies were supported by grants from the Heart and Stroke Foundation of Alberta, Northwest Territories and Nunavut, the Canadian Institutes for Health Research to R. Loutzenhiser and a Visiting Scientist Award from the Alberta Heritage Foundation for Medical Research (AHFMR) to P. I. Aaronson. R. Loutzenhiser is an AHFMR Scientist.
Acknowledgments
We thank Dr. Mike Walsh for comments and suggestions.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bell PD, Mashburn N, Unlap MT. Renal sodium/calcium exchange: a vasodilator that is defective in salt-sensitive hypertension. Acta Physiol Scand 168: 209–214, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Benos DJ, Cunningham S, Baker RR, Beason KB, Smith PR. Molecular characteristics of amiloride-sensitive sodium channels. Rev Physiol Biochem Pharmacol 120: 31–113, 1992. [DOI] [PubMed] [Google Scholar]
- 3.Bertorello AM, Katz AI. Short-term regulation of renal Na+-K+-ATPase activity: physiological relevance and cellular mechanisms. Am J Physiol Renal Fluid Electrolyte Physiol 265: F743–F755, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Casteels R, Kitamura K, Kuriyama H, Suzuki H. The membrane properties of the smooth muscle cells of the rabbit main pulmonary artery. J Physiol 271: 41–61, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chilton L, Loutzenhiser R. Functional evidence for an inward rectifier potassium current in rat renal afferent arterioles. Circ Res 88:152–158, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Cupples WA, Loutzenhiser R. Dynamic autoregulation in the in vitro perfused hydronephrotic kidney. Am J Physiol Renal Physiol 275: F126–F130, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Drummond HA, Gebremedhin D, Harder DR. Degenerin/epithelial Na+ channel proteins: components of a vascular mechanosensor. Hypertension 44: 643–648, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Ducoudret O, Diakov A, Müller-Berger S, Romero MF, Frömter E. The renal Na-HCO3−cotransporter expressed in Xenopus laevis oocytes: inhibition by tenidap and benzamil and effect of temperature on transport rate and stoichiometry. Pflugers Arch 442: 709–717, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Ehrenfeld J, Lacoste I, Harvey BJ. Effects of intracellular signals on Na+/K+-ATPase pump activity in the frog skin epithelium. Biochim Biophys Acta 1106: 197–208, 1992. [DOI] [PubMed] [Google Scholar]
- 10.Fowler BC, Carmines PK, Nelson LD, Bell PD. Characterization of sodium-calcium exchange in rabbit renal arterioles. Kidney Int 50: 1856–1862, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Gormley K, Dong Y, Sagnella GA. Regulation of the epithelial sodium channel by accessory proteins. Biochem J 371: 1–14, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hager H, Kwon TH, Vinnikova AK, Masilamani S, Brooks HL, Frökiaer J, Knepper MA, Nielsen S. Immunocytochemical and immunoelectron microscopic localization of α-, β-, and γ-ENaC in rat kidney. Am J Physiol Renal Physiol 280: F1093–F1106, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi K, Epstein M, Loutzenhiser R. Pressure-induced vasoconstriction of renal microvessels in normotensive and hypertensive rats: studies in the isolated perfused hydronephrotic kidney. Circ Res 65: 475–1484, 1989. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi K, Epstein M, Loutzenhiser R. Determinants of the renal actions of atrial natriuretic peptide (A.N.P.): lack of effect on A.N.P. on pressure-induced vasoconstriction. Circ Res. 67: 1–10, 1990. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi K, Epstein M, Loutzenhiser R, Forster H. Impaired myogenic responsiveness of the renal afferent arteriole in streptozotocin-induced diabetic rats: role of eicosanoid derangements. J Am Soc Nephrol 2: 1578–1586, 1992. [DOI] [PubMed] [Google Scholar]
- 16.Jernigan NL, Drummond HA. Vascular ENaC proteins are required for renal myogenic constriction. Am J Physiol Renal Physiol 289: F891–F901, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Jernigan NL, Drummond HA. Myogenic vasoconstriction in mouse renal interlobar arteries:role of endogenous beta and gamma ENaC. Am J Physiol Renal Physiol 291: F1184–F1191, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Jernigan NL, La Marca B, Speed J, Galmiche L, Granger JP, Drummond HA. Dietary salt enhances benzamil sensitive component of myogenic constriction in mesenteric arteries. Am J Physiol Heart Circ Physiol 294: H409–H420, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Kirton CA, Loutzenhiser R. Alterations in basal PKC activity modulate renal afferent arteriolar myogenic reactivity. Am J Physiol Heart Circ Physiol 275: H467–H475, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Kleyman TR, Cragoe EJ Jr. Amiloride and its analogs as tools in the study of ion transport. J Membr Biol 105: 1–21, 1988. [DOI] [PubMed] [Google Scholar]
- 21.Kusche-Vihrog K, Sobczak K, Bangel N, Wilhelmi M, Nechyporuk-Zloy V, Schwab A, Schillers H, Oberleithner H. Aldosterone and amiloride alter ENaC abundance in vascular endothelium. Pflugers Arch 455: 849–857, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Li C, Wang W, Norregaard R, Knepper MA, Nielsen S, Frökiaer J. Altered expression of epithelial sodium channel in rats with bilateral or unilateral ureteral obstruction. Am J Physiol Renal Physiol 293: F333–F341, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Loutzenhiser R, Bidani A, Chilton L. The renal myogenic response: kinetic attributes and physiologic role. Circ Res 90: 1316–1324, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Loutzenhiser R, Chilton L, Trottier G. Membrane potential measurements in renal afferent and efferent arterioles: actions of angiotensin II. Am J Physiol Renal Physiol 273: F307–F314, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Loutzenhiser R, Epstein M, Horton C. Inhibition by diltiazem of pressure-induced afferent vasoconstriction of the isolated perfused rat kidney. Am J Cardiol 59: 72A–75A, 1987. [DOI] [PubMed] [Google Scholar]
- 26.Loutzenhiser R, Griffin KA, Williamson GA, Bidani AK. Renal autoregulation: new perspectives regarding protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol 290: R1153–R1166, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loutzenhiser K, Loutzenhiser R. Angiotensin II-induced Ca2+ influx in renal afferent and efferent arterioles. Differing roles of voltage-gated and store-operated Ca2+ entry. Circ Res 87: 551–557, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura Y, Ohya Y, Abe I, Fujishima M. Sodium-potassium pump current in smooth muscle cells from mesenteric resistance arteries of the guinea-pig. J Physiol 519: 203–212, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neher E Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol 207: 123–131, 1992. [DOI] [PubMed] [Google Scholar]
- 30.Nelson LD, Mashburn NA, Bell PD. Altered sodium-calcium exchange in afferent arterioles of the spontaneously hypertensive rat. Kidney Int 50: 1889–1896, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Pallone TL, Huang JM. Control of descending vasa recta pericyte membrane potential by angiotensin II. Am J Physiol Renal Physiol 282: F1064–F1074, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Quinn K, Guibert C, Beech DJ. Sodium-potassium-ATPase electrogenicity in cerebral precapillary arterioles. Am J Physiol Heart Circ Physiol 279: H351–H360, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Schweda F, Kramer BK, Kurtz A. Differential roles of the sodium-calcium exchanger in renin secretion and renal vascular resistance. Pflugers Arch 442: 693–699, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Schweda F, Seebauer H, Kramer BK, Kurtz A. Functional role of sodium-calcium exchange in the regulation of renal vascular resistance. Am J Physiol Renal Physiol 280: F155–F161, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Sejersted OM, Wasserstrom JA, Fozzard HA. Na+,K+ pump stimulation by intracellular Na+ in isolated, intact sheep cardiac Purkinje fibers. J Gen Physiol 91: 445–466, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takenaka T, Forster H, De Micheli A, Epstein M. Impaired myogenic responsiveness of renal microvessels in Dahl salt-sensitive rats. Circ Res 71: 471–480, 1992. [DOI] [PubMed] [Google Scholar]
- 37.Tang L, Loutzenhiser K, Loutzenhiser R. Biphasic actions of PGE2 on the renal afferent arteriole, role of EP3 and EP4 receptors. Circ Res 86: 663–670, 2000. [DOI] [PubMed] [Google Scholar]
- 38.van Rodijnen WF, van Lambalgen TA, Tangelder GJ, van Dokkum RP, Provoost AP, ter Wee PM. Reduced reactivity of renal microvessels to pressure and angiotensin II in fawn-hooded rats. Hypertension 39: 111–115, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Waters A, Harder DR. Altered membrane properties of cerebral vascular smooth muscle following subarachnoid hemorrhage: an electrophysiological study. I. Changes in resting membrane potential (Em) and effect on the electrogenic pump potential contribution to Em. Stroke 16: 990–997, 1985. [DOI] [PubMed] [Google Scholar]