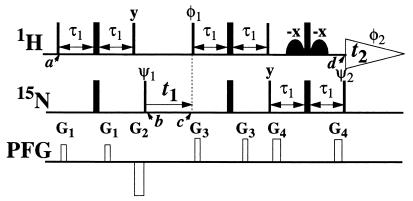
Figure 1.
Experimental scheme for TROSY-type two-dimensional 1H,15N correlation spectroscopy. In the rows marked 1H and 15N, narrow and wide bars stand for nonselective 90° and 180° rf-pulses, respectively. Water suppression is achieved by watergate (34), using the two off-resonance rf-pulses indicated by curved shapes. The 1H and 15N carrier frequencies are placed at 9 and 127 ppm, respectively. The delay τ1 corresponds to 1/(41J(1H,15N)) = 2.7 ms. Phases used are ψ1 = {y,−y,−x,x,y,−y,−x,x}; ψ2 = {4(x),4(-x)}; φ1 = {4(y),4(-y)}; φ2 (receiver) = {x,−x,−y,y,x,−x,y,−y}; x on all other pulses. The row marked PFG (pulsed field gradient) indicates the applied magnetic field gradients along the z-axis: G1, amplitude = 30 G/cm, duration = 0.4 ms; G2, −60 G/cm, 1 ms; G3, 50 G/cm, 0.4 ms; G4, 48 G/cm, 0.6 ms. Two free induction decays are recorded per t1 delay, with ψ1 incremented by 90° in between and stored as the real and imaginary parts of the interferogram in t1. The Fourier transformation results in a two-dimensional 1H,15N correlation spectrum that contains only the component of the four-line 15N–1H multiplet that has the slowest T2 relaxation rates for both nuclei. With this scheme, DD/CSA relaxation interference, which has been known for many years (35, 36), can be used to extend the limits of protein NMR.