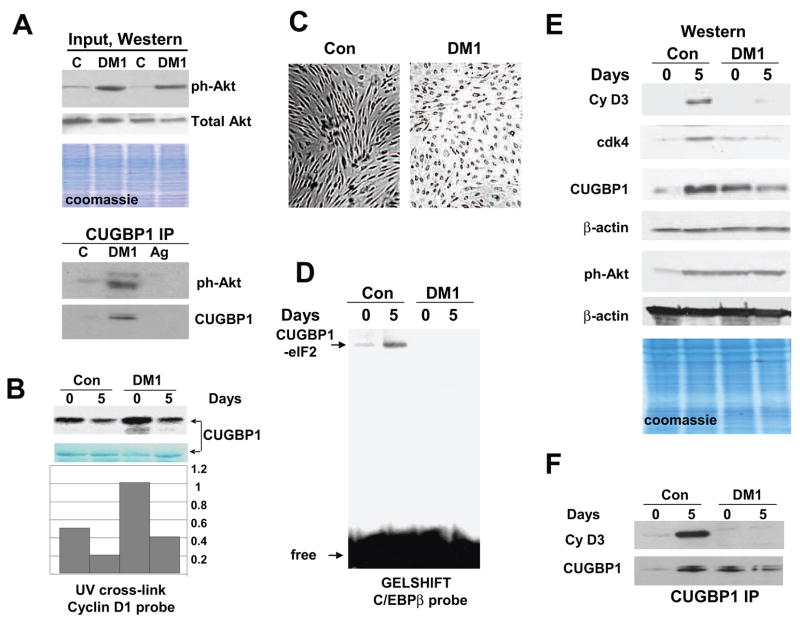
Figure 4. Mis-regulation of Akt-CUGBP1 and cyclinD3/cdk-CUGBP1 pathways in DM1 myogenesis.
A. Input. The levels of active ph-Akt and total Akt in cytoplasm used for the IP. Western blotting of cytoplasmic extracts from control and DM1 myoblasts was performed with ph-Akt antibodies and with antibodies to total Akt. Cells from two control and two DM1 patients were analyzed. Bottom image shows coomassie staining of the membrane. CUGBP1-IP: Interactions of “active” Akt with CUGBP1 are increased in DM1 myoblasts. CUGBP1 was precipitated from control and DM1 myoblasts and ph-Akt was detected by Western blotting. Ag; mock agarose control. B. Incubation of bacterially expressed CUGBP1 with cytoplasmic proteins from DM1 cells increases interactions of CUGBP1 with cyclin D1 mRNA. CUGBP1 was incubated with cytoplasmic proteins from control and DM1 cells in the presence of 1mM ATP and examined by UV cross-link with cyclin D1 probe. The filter was stained with coomassie. Bar graphs show the ratio of CUGBP1 signals on UV cross link film to signals of CUGBP1 detected by coomassie stain. C. Delay of differentiation of DM1 cells. Bright field microscopy shows cells differentiating for 5 days. Cells were derived from control and DM1 patient. D. Amounts of the CUGBP1-eIF2 complex are reduced in DM1 differentiating cells. Gelshift was performed with cytoplasmic protein extracts isolated from control and DM1 proliferating and differentiating cells using C/EBPβ probe. Positions of the CUGBP1-eIF2 complex and free probe are shown by arrows. E. Mis-regulation of Akt and cyclin D3/cdk4 pathways in DM1 myogenesis. Cyclin D3 and cdk4 levels are reduced in DM1 differentiating cells while levels of active Akt remain high during proliferation and differentiation of DM1 muscle cells. Cytoplasmic proteins from control and DM1 proliferating and differentiating cells were analyzed by Western blotting with antibodies to cyclin D3, cdk4, CUGBP1 and ph-Akt. The membranes were re-probed with antibodies to β-actin as a loading control. Bottom image shows a coomassie stain of the membrane. F. Cyclin D3 does not interact with CUGBP1 in DM1 cells during differentiation. CUGBP1 was immunoprecipitated from control and DM1 cytoplasm. The IPs were probed with antibodies to cyclin D3 and to CUGBP1.