Abstract
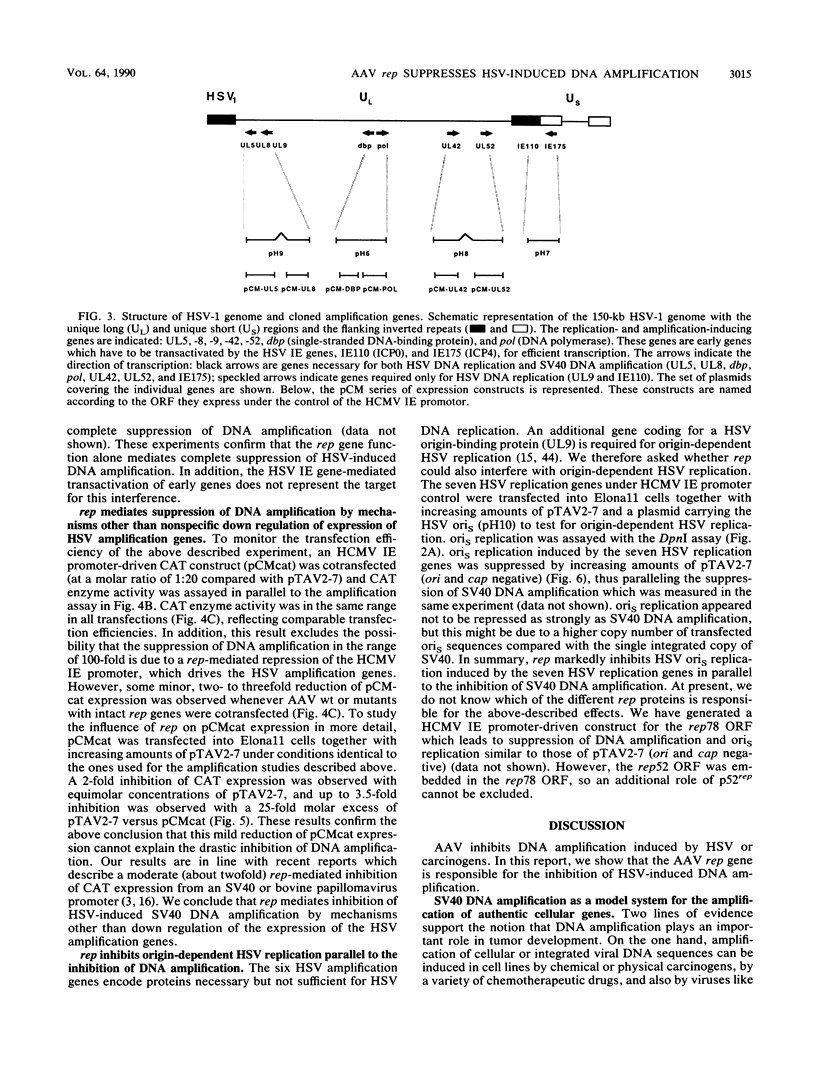
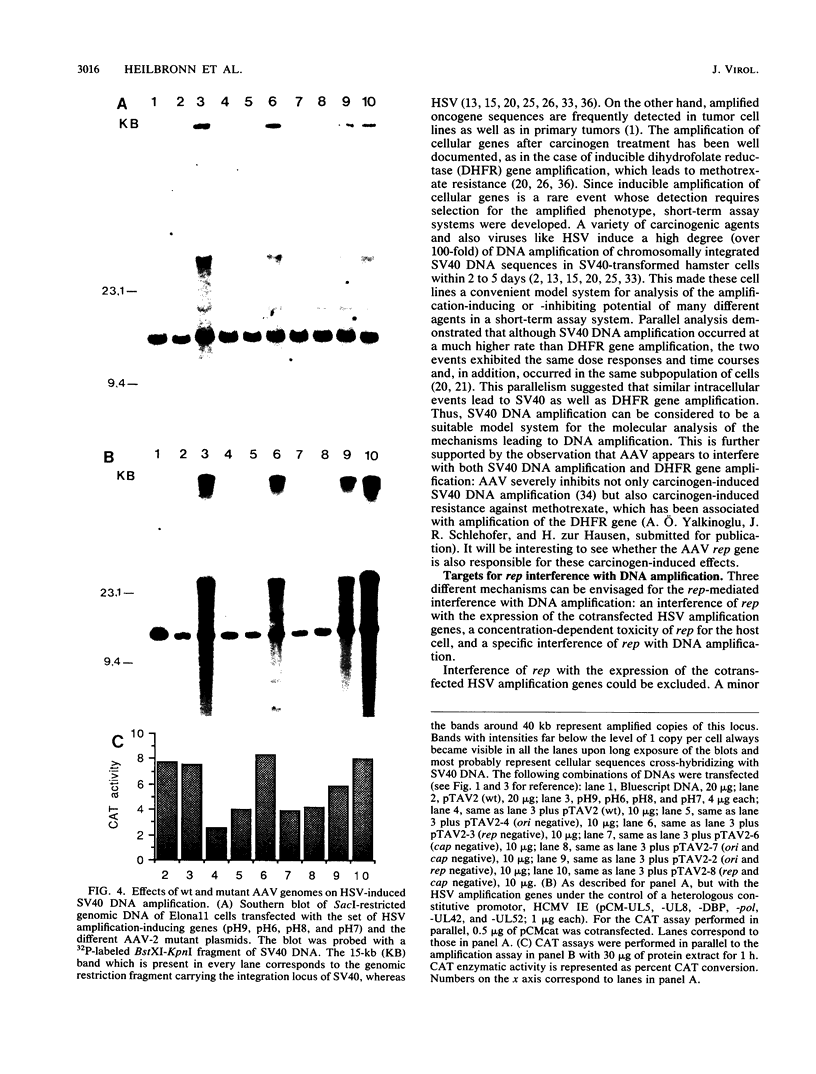
Herpes simplex virus (HSV) induces within the host cell genome DNA amplification which can be suppressed by coinfection with adeno-associated virus (AAV). To characterize the AAV functions mediating this effect, cloned AAV type 2 wild-type or mutant genomes were transfected into simian virus 40 (SV40)-transformed hamster cells together with the six HSV replication genes (encoding UL5, UL8, major DNA-binding protein, DNA polymerase, UL42, and UL52) which together are necessary and sufficient for the induction of SV40 DNA amplification (R. Heilbronn and H. zur Hausen, J. Virol. 63:3683-3692, 1989). The AAV rep gene was identified as being responsible for the complete inhibition of HSV-induced SV40 DNA amplification. Likewise, rep inhibited origin-dependent HSV replication. rep neither killed the transfected host cells nor interfered with gene expression from the cotransfected amplification genes. This points to a specific interference with HSV-induced DNA amplification.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Schwab M. Oncogene amplification in tumor cells. Adv Cancer Res. 1986;47:235–281. doi: 10.1016/s0065-230x(08)60201-8. [DOI] [PubMed] [Google Scholar]
- Bantel-Schaal U., zur Hausen H. Adeno-associated viruses inhibit SV40 DNA amplification and replication of herpes simplex virus in SV40-transformed hamster cells. Virology. 1988 May;164(1):64–74. doi: 10.1016/0042-6822(88)90620-4. [DOI] [PubMed] [Google Scholar]
- Beaton A., Palumbo P., Berns K. I. Expression from the adeno-associated virus p5 and p19 promoters is negatively regulated in trans by the rep protein. J Virol. 1989 Oct;63(10):4450–4454. doi: 10.1128/jvi.63.10.4450-4454.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K. I., Labow M. A. Parvovirus gene regulation. J Gen Virol. 1987 Mar;68(Pt 3):601–614. doi: 10.1099/0022-1317-68-3-601. [DOI] [PubMed] [Google Scholar]
- Casto B. C., Goodheart C. R. Inhibition of adenovirus transformation in vitro by AAV-1. Proc Soc Exp Biol Med. 1972 May;140(1):72–78. doi: 10.3181/00379727-140-36397. [DOI] [PubMed] [Google Scholar]
- Chejanovsky N., Carter B. J. Mutagenesis of an AUG codon in the adeno-associated virus rep gene: effects on viral DNA replication. Virology. 1989 Nov;173(1):120–128. doi: 10.1016/0042-6822(89)90227-4. [DOI] [PubMed] [Google Scholar]
- Cheung A. K., Hoggan M. D., Hauswirth W. W., Berns K. I. Integration of the adeno-associated virus genome into cellular DNA in latently infected human Detroit 6 cells. J Virol. 1980 Feb;33(2):739–748. doi: 10.1128/jvi.33.2.739-748.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb D. W., Dixon J. E. A method for increasing the sensitivity of chloramphenicol acetyltransferase assays in extracts of transfected cultured cells. Anal Biochem. 1987 May 15;163(1):88–92. doi: 10.1016/0003-2697(87)90096-0. [DOI] [PubMed] [Google Scholar]
- Cukor G., Blacklow N. R., Kibrick S., Swan I. C. Effect of adeno-associated virus on cancer expression by herpesvirus-transformed hamster cells. J Natl Cancer Inst. 1975 Oct;55(4):957–959. doi: 10.1093/jnci/55.4.957. [DOI] [PubMed] [Google Scholar]
- Georg-Fries B., Biederlack S., Wolf J., zur Hausen H. Analysis of proteins, helper dependence, and seroepidemiology of a new human parvovirus. Virology. 1984 Apr 15;134(1):64–71. doi: 10.1016/0042-6822(84)90272-1. [DOI] [PubMed] [Google Scholar]
- Handa H., Shiroki K., Shimojo H. Establishment and characterization of KB cell lines latently infected with adeno-associated virus type 1. Virology. 1977 Oct 1;82(1):84–92. doi: 10.1016/0042-6822(77)90034-4. [DOI] [PubMed] [Google Scholar]
- Heilbronn R., Schlehofer J. R., Yalkinoglu A. O., Zur Hausen H. Selective DNA-amplification induced by carcinogens (initiators): evidence for a role of proteases and DNA polymerase alpha. Int J Cancer. 1985 Jul 15;36(1):85–91. doi: 10.1002/ijc.2910360114. [DOI] [PubMed] [Google Scholar]
- Heilbronn R., Schlehofer J. R., zur Hausen H. Selective killing of carcinogen-treated SV40-transformed Chinese hamster cells by a defective parvovirus. Virology. 1984 Jul 30;136(2):439–441. doi: 10.1016/0042-6822(84)90180-6. [DOI] [PubMed] [Google Scholar]
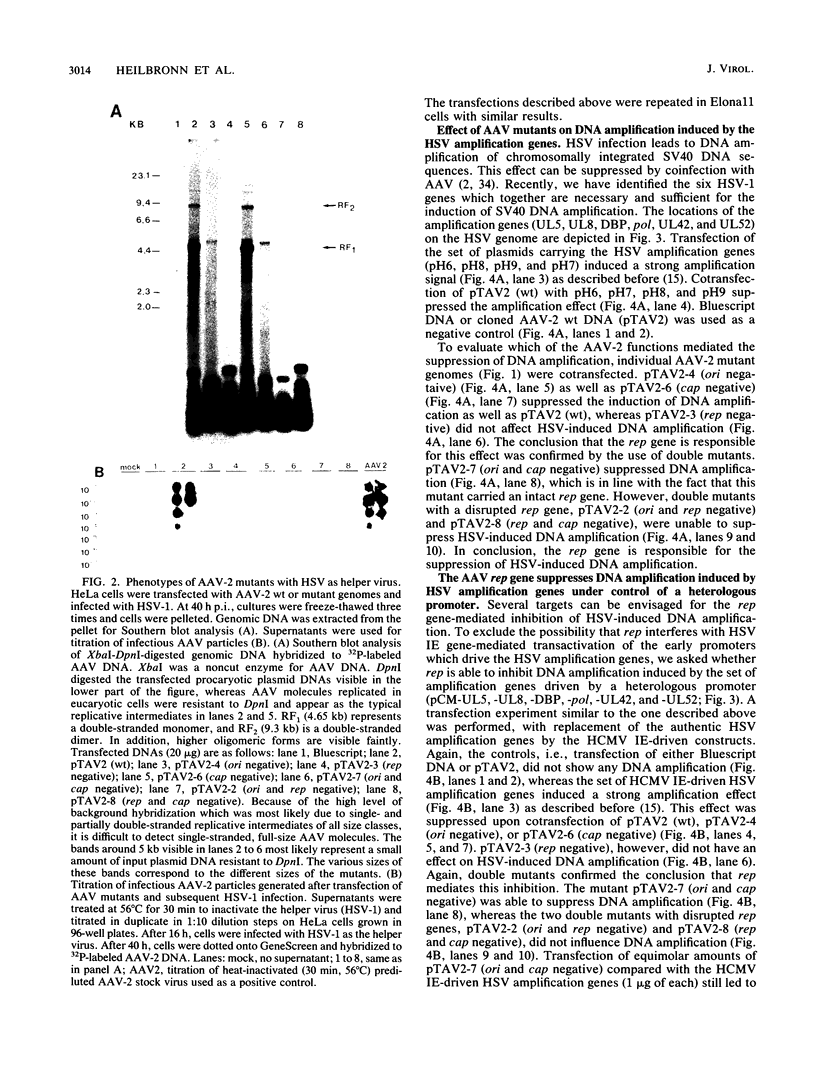
- Heilbronn R., zur Hausen H. A subset of herpes simplex virus replication genes induces DNA amplification within the host cell genome. J Virol. 1989 Sep;63(9):3683–3692. doi: 10.1128/jvi.63.9.3683-3692.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermonat P. L., Labow M. A., Wright R., Berns K. I., Muzyczka N. Genetics of adeno-associated virus: isolation and preliminary characterization of adeno-associated virus type 2 mutants. J Virol. 1984 Aug;51(2):329–339. doi: 10.1128/jvi.51.2.329-339.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermonat P. L. The adeno-associated virus Rep78 gene inhibits cellular transformation induced by bovine papillomavirus. Virology. 1989 Sep;172(1):253–261. doi: 10.1016/0042-6822(89)90127-x. [DOI] [PubMed] [Google Scholar]
- Im D. S., Muzyczka N. Factors that bind to adeno-associated virus terminal repeats. J Virol. 1989 Jul;63(7):3095–3104. doi: 10.1128/jvi.63.7.3095-3104.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschstein R. L., Smith K. O., Peters E. A. Inhibition of adenovirus 12 oncogenicity by adeno-associated virus. Proc Soc Exp Biol Med. 1968 Jul;128(3):670–673. doi: 10.3181/00379727-128-33095. [DOI] [PubMed] [Google Scholar]
- Kleinberger T., Etkin S., Lavi S. Carcinogen-mediated methotrexate resistance and dihydrofolate reductase amplification in Chinese hamster cells. Mol Cell Biol. 1986 Jun;6(6):1958–1964. doi: 10.1128/mcb.6.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberger T., Sahar E., Lavi S. Carcinogen-mediated co-activation of two independent genes in Chinese hamster cells. Carcinogenesis. 1988 Jun;9(6):979–985. doi: 10.1093/carcin/9.6.979. [DOI] [PubMed] [Google Scholar]
- Labow M. A., Berns K. I. The adeno-associated virus rep gene inhibits replication of an adeno-associated virus/simian virus 40 hybrid genome in cos-7 cells. J Virol. 1988 May;62(5):1705–1712. doi: 10.1128/jvi.62.5.1705-1712.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labow M. A., Graf L. H., Jr, Berns K. I. Adeno-associated virus gene expression inhibits cellular transformation by heterologous genes. Mol Cell Biol. 1987 Apr;7(4):1320–1325. doi: 10.1128/mcb.7.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin C. A., Tratschin J. D., Coon H., Carter B. J. Cloning of infectious adeno-associated virus genomes in bacterial plasmids. Gene. 1983 Jul;23(1):65–73. doi: 10.1016/0378-1119(83)90217-2. [DOI] [PubMed] [Google Scholar]
- Lavi S. Carcinogen-mediated amplification of viral DNA sequences in simian virus 40-transformed Chinese hamster embryo cells. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6144–6148. doi: 10.1073/pnas.78.10.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. C., Tanaka N., Lamb P. W., Gilmer T. M., Barrett J. C. Induction of gene amplification by arsenic. Science. 1988 Jul 1;241(4861):79–81. doi: 10.1126/science.3388020. [DOI] [PubMed] [Google Scholar]
- Mayor H. D., Drake S., Stahmann J., Mumford D. M. Antibodies to adeno-associated satellite virus and herpes simplex in sera from cancer patients and normal adults. Am J Obstet Gynecol. 1976 Sep 1;126(1):100–104. doi: 10.1016/0002-9378(76)90472-5. [DOI] [PubMed] [Google Scholar]
- Mayor H. D., Houlditch G. S., Mumford D. M. Influence of adeno-associated satellite virus on adenovirus-induced tumours in hamsters. Nat New Biol. 1973 Jan 10;241(106):44–46. doi: 10.1038/newbio241044b0. [DOI] [PubMed] [Google Scholar]
- Mousset S., Rommelaere J. Minute virus of mice inhibits cell transformation by simian virus 40. Nature. 1982 Dec 9;300(5892):537–539. doi: 10.1038/300537a0. [DOI] [PubMed] [Google Scholar]
- Ostrove J. M., Duckworth D. H., Berns K. I. Inhibition of adenovirus-transformed cell oncogenicity by adeno-associated virus. Virology. 1981 Sep;113(2):521–533. doi: 10.1016/0042-6822(81)90180-x. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Ayub J., Kajigaya S., Shimada T., Young N. The gene encoding the nonstructural protein of B19 (human) parvovirus may be lethal in transfected cells. J Virol. 1988 Aug;62(8):2884–2889. doi: 10.1128/jvi.62.8.2884-2889.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Construction of a genetic switch for inducible trans-activation of gene expression in eucaryotic cells. J Virol. 1987 May;61(5):1448–1456. doi: 10.1128/jvi.61.5.1448-1456.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlehofer J. R., Gissmann L., Matz B., zur Hausen H. Herpes simplex virus-induced amplification of SV40 sequences in transformed Chinese hamster embryo cells. Int J Cancer. 1983 Jul 15;32(1):99–103. doi: 10.1002/ijc.2910320116. [DOI] [PubMed] [Google Scholar]
- Schlehofer J. R., Heilbronn R., Georg-Fries B., zur Hausen H. Inhibition of initiator-induced SV40 gene amplification in SV40-transformed Chinese hamster cells by infection with a defective parvovirus. Int J Cancer. 1983 Nov 15;32(5):591–595. doi: 10.1002/ijc.2910320512. [DOI] [PubMed] [Google Scholar]
- Sprecher-Goldberger S., Thiry L., Lefébvre N., Dekegel D., de Halleux F. Complement-fixation antibodies to adenovirus-associated viruses, cytomegaloviruses and herpes simplex viruses in patients with tumors and in control individuals. Am J Epidemiol. 1971 Oct;94(4):351–358. doi: 10.1093/oxfordjournals.aje.a121330. [DOI] [PubMed] [Google Scholar]
- Tlsty T. D., Brown P. C., Schimke R. T. UV radiation facilitates methotrexate resistance and amplification of the dihydrofolate reductase gene in cultured 3T6 mouse cells. Mol Cell Biol. 1984 Jun;4(6):1050–1056. doi: 10.1128/mcb.4.6.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toolan H. W. Lack of oncogenic effect of the H-viruses for hamsters. Nature. 1967 Jun 3;214(5092):1036–1036. doi: 10.1038/2141036a0. [DOI] [PubMed] [Google Scholar]
- Toolan H. W., Ledinko N. Inhibition by H-1 virus of the incidence of tumors produced by adenovirus 12 in hamsters. Virology. 1968 Jul;35(3):475–478. doi: 10.1016/0042-6822(68)90226-2. [DOI] [PubMed] [Google Scholar]
- Toolan H. W., Rhode S. L., 3rd, Gierthy J. F. Inhibition of 7,12-dimethylbenz(a)anthracene-induced tumors in Syrian hamsters by prior infection with H-1 parvovirus. Cancer Res. 1982 Jul;42(7):2552–2555. [PubMed] [Google Scholar]
- Tratschin J. D., Miller I. L., Carter B. J. Genetic analysis of adeno-associated virus: properties of deletion mutants constructed in vitro and evidence for an adeno-associated virus replication function. J Virol. 1984 Sep;51(3):611–619. doi: 10.1128/jvi.51.3.611-619.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tratschin J. D., Tal J., Carter B. J. Negative and positive regulation in trans of gene expression from adeno-associated virus vectors in mammalian cells by a viral rep gene product. Mol Cell Biol. 1986 Aug;6(8):2884–2894. doi: 10.1128/mcb.6.8.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempe J. P., Carter B. J. Regulation of adeno-associated virus gene expression in 293 cells: control of mRNA abundance and translation. J Virol. 1988 Jan;62(1):68–74. doi: 10.1128/jvi.62.1.68-74.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocour E., Callaham M. F., Huberman E. Perturbation of the cell cycle by adeno-associated virus. Virology. 1988 Dec;167(2):393–399. [PubMed] [Google Scholar]
- Wu C. A., Nelson N. J., McGeoch D. J., Challberg M. D. Identification of herpes simplex virus type 1 genes required for origin-dependent DNA synthesis. J Virol. 1988 Feb;62(2):435–443. doi: 10.1128/jvi.62.2.435-443.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakobson B., Koch T., Winocour E. Replication of adeno-associated virus in synchronized cells without the addition of a helper virus. J Virol. 1987 Apr;61(4):972–981. doi: 10.1128/jvi.61.4.972-981.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalkinoglu A. O., Heilbronn R., Bürkle A., Schlehofer J. R., zur Hausen H. DNA amplification of adeno-associated virus as a response to cellular genotoxic stress. Cancer Res. 1988 Jun 1;48(11):3123–3129. [PubMed] [Google Scholar]
- de la Maza L. M., Carter B. J. Inhibition of adenovirus oncogenicity in hamsters by adeno-associated virus DNA. J Natl Cancer Inst. 1981 Dec;67(6):1323–1326. [PubMed] [Google Scholar]