Figure 3. The P58S mutation leads to a failure to secrete dVAP and forms ubiquitinated inclusions.
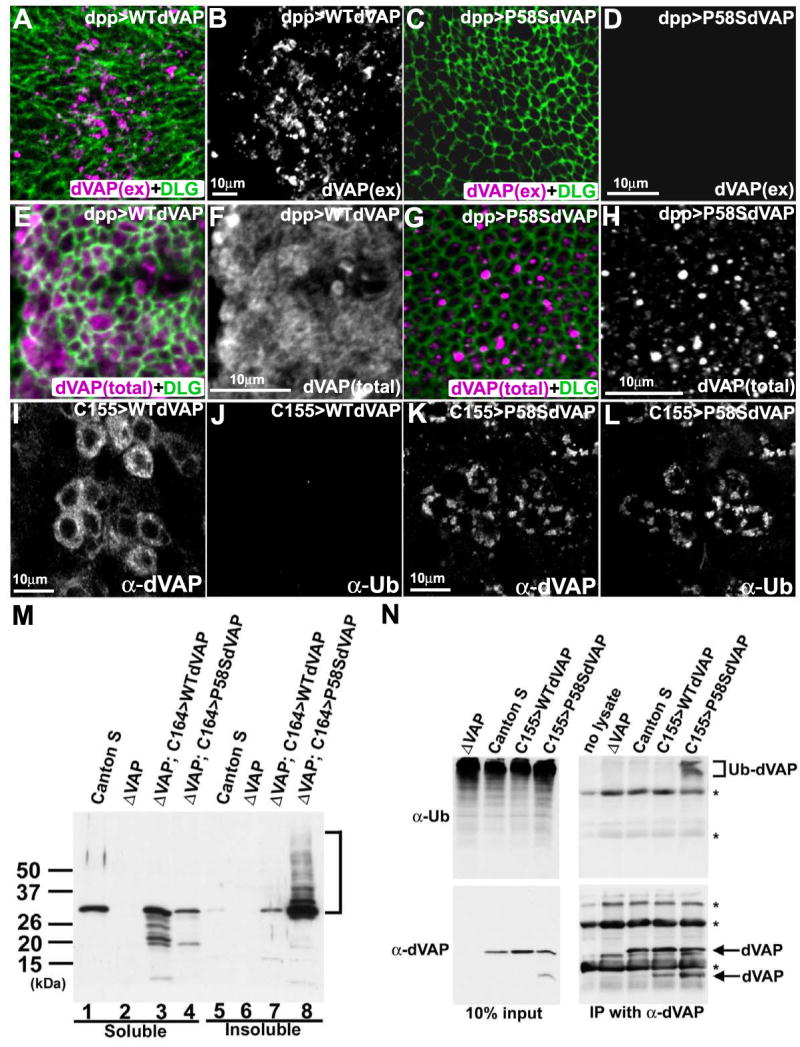
(A-D) The P58S mutation leads to a loss of the extracellular localization of dVAP. The wing discs expressing WT dVAP (A, B) and P58S dVAP (C, D) were stained using the anti-dVAP (magenta) extracellular staining protocol, and subsequently stained with anti-DLG (green) upon permeabilization.
(E-H) The P58S mutation causes dVAP protein to be localized as intracellular inclusions. Wing discs expressing WT dVAP (E, F) and P58S dVAP (G, H).
(I-L) Expression of P58S dVAP causes ubiquitin-containing inclusions. Neurons expressing WT dVAP (I, J) or P58S dVAP (K, L) were stained with anti-dVAP (I, K) and anti-Ubiquitin (J, L). The laser intensity was adjusted for the signals of overexpressed proteins. Hence, endogenous expression of dVAP cannot be observed (Figure 3A-L).
(M) The P58S mutation causes detergent insoluble aggregates. Extracts of Canton S, null mutant (ΔVAP), and null mutant flies overexpressing WT or P58S dVAP were immunoblotted with anti-dVAP. Detergent soluble fractions (lane1-4); insoluble fractions (lane 5-8).
(N) P58S dVAP is ubiquitinated. Anti-dVAP (GP33) was used to immunoprecipitate dVAP from extracts of ΔVAP and flies expressing WT (C155>WTdVAP) or P58S dVAP (C155>P58SdVAP). Immunoblots of anti-Ubiqutin (right top panel) and anti-dVAP (right bottom panel). (bracket) ubiquitin positive high molecular weight products * non-specific signals.
