Figure 7. VAP MSP domains bind to Eph receptor extracellular domains.
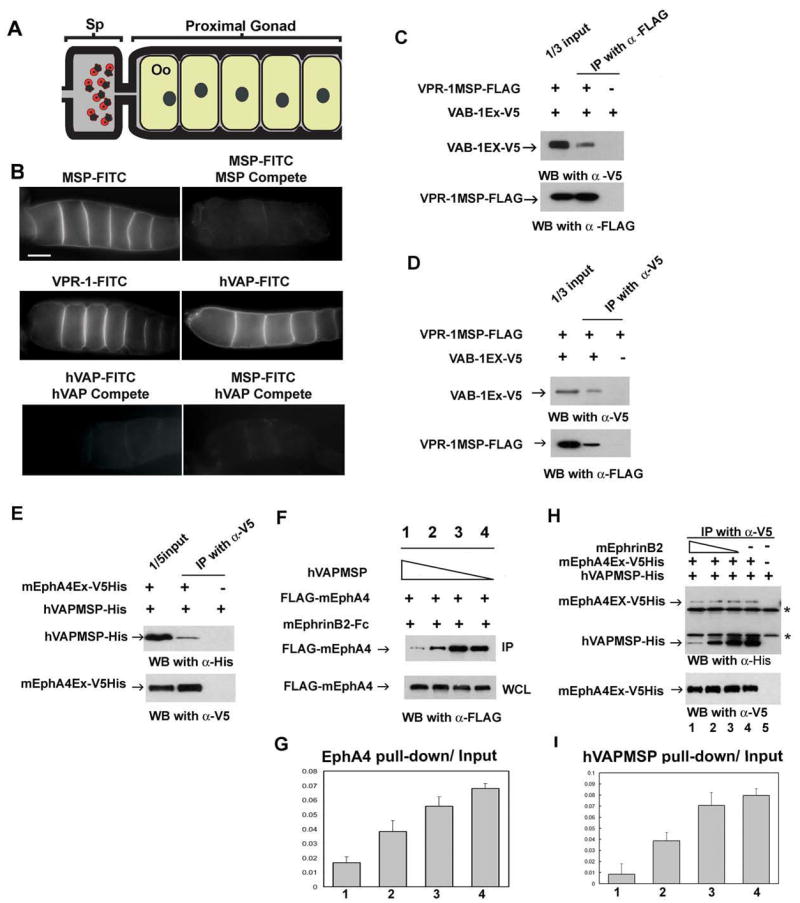
(A) Diagram of the C. elegans proximal gonad. MSP binds to receptors on the oocyte (Oo) and sheath cell surfaces in the proximal gonad (Miller et al. 2003; Corrigan et al. 2005).
(B) Receptor binding sites are visualized using FITC-conjugated MSP domains. FITC-conjugates are biological active in promoting oocyte maturation and sheath contraction (Miller et al. 2003; data not shown). Compete includes a 25-fold molar excess of unlabelled protein. Bar, 20 μm.
(C, D) VPR-1 MSP domain can bind to VAB-1 Eph receptor. Purified FLAG-tagged VPR-1 MSP (VPR-1MSP-FLAG) and V5 tagged VAB-1 ECT domain (VAB-1 Ex-V5) were co-immunoprecipitated using FLAG antibody (C) and V5 antibody (D). IP, immunoprecipitation.
(E) His- and V5- tagged mouse EphA4 ectodomain (mEphA4Ex-V5His) and a His tagged hVAPB MSP (hVAPMSP-His) co-immunoprecipitate using anti-V5 antibody.
(F) Conditioned medium containing hVAPMSP disrupts the interaction between mouse EphrinB2 (mEphrinB2) and EphA4. WCL: whole cell lysates.
(G) Quantification of the fraction of IP of (F). Lanes 1 to 4 correspond to the lanes in Figure 7F.
(H) Ephrin B2 competes with hVAPB MSP for binding of EphA4 in a dose dependent manner. As decreasing amounts of EphrinB2 are added, increasing amounts of hVAP MSP are pulled down by mEphA4Ex-V5 His. Note that EphrinB2-His cannot be detected in the top panel because it has a similar molecular weight to the IgG light chain. * shows non-specific heavy and light chains of IgG.
(I) The quantification of the fraction of IP of (H). The lanes correspond to the lanes 1 to 4 in Figure 7H.
