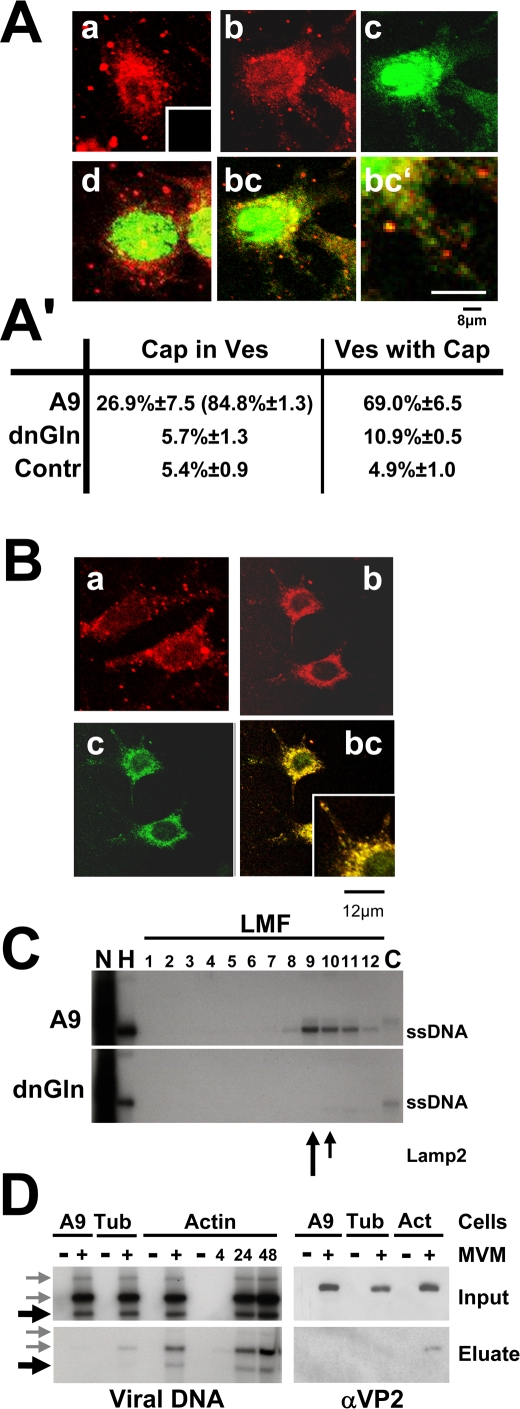
Figure 5. Vesicular transport of MVM progeny virions.
(A, B) A9 cells and derivatives expressing a mutant gelsolin were infected (or not) with CsCl-gradient-purified MVM (30 pfu/cell), treated with neuraminidase to avoid second-round infection, and fixed at 24 h p.i. Capsids (detected with αB7 [green]) were analyzed by confocal laser scanning and spinning-disk microscopy for their subcellular localization, as compared to that of Lamp2-positive vesicles. Progeny virions are associated with cellular lysosomes and/or late endosomes as apparent from colocalization with the vesicular marker Lamp2 in the cytosol. (A) a, mock-treated A9 cells; Lamp2 (main panel) and the negative control for capsid staining (insert); b, Lamp2 staining of MVM-infected A9 cells; c, MVM capsids in infected A9 cells; bc, Lamp2/capsid merge; bc′, enlarged area; d, Lamp2/capsid merge applied to MVM-infected A9 cells expressing a dominant-negative gelsolin variant. Scale bars: 8 µm. (A′) Capsid/Lamp2 colocalization determined with imageJ, expressed as the mean value of a whole stack. Capsid/mitotracker (suppl. 3) served as a negative control (Contr.). Cap in Ves, percentage of green pixels merging with red. Values in parentheses are derived from cytoplasmic areas; Ves with Cap, red pixels merging with green. (B) a, dynamin in mock-treated A9; b, dynamin in MVM-infected A9; c, MVM capsids; bc, dynamin/capsid merge. Small squares represent enlarged areas of capsid/dynamin colocalization. Scale bars 12 µm. (C) A9 cells and cells of the derivative expressing GlnY435A were infected with MVM (30 pfu/cell), harvested at 24 h p.i., and fractionated by differential (density) centrifugation to separate different organelles. The presence of progeny particles was determined by Southern blotting (revealing their single-stranded DNA). DNA-containing progeny virions co-purify with cellular vesicles during biochemical fractionations of cellular organelles. Nuc, purified nuclei; HMF, large organelles; Cyt, cytosol. Cellular vesicles were further purified from the light mitochondrial fraction (LMF) by centrifugation through an iodixanol gradient. The migration of Lamp2 is indicated by arrows. (D) A9 cells (lanes 1&2) and derivatives expressing either GST-tagged α-tubulin (lanes 3 and 4) or β-actin (lanes 5–10) were mock-treated (−) or infected with MVM for the indicated time (h). Cell extracts were prepared and run through Glutathione Sepharose columns specifically retaining the GST-tagged proteins and their associated partners. The partners were recovered (700 mM NaCl Eluate) and tested for the presence of full (virion-containing) capsids by Southern blotting (ssDNA) and Western blotting (capsid proteins), by comparison with the corresponding total extracts (Input). Black arrows indicate the migration of ssDNA, grey arrows of free replicative form viral DNAs. DNA-containing progeny particles specifically interact with actin.