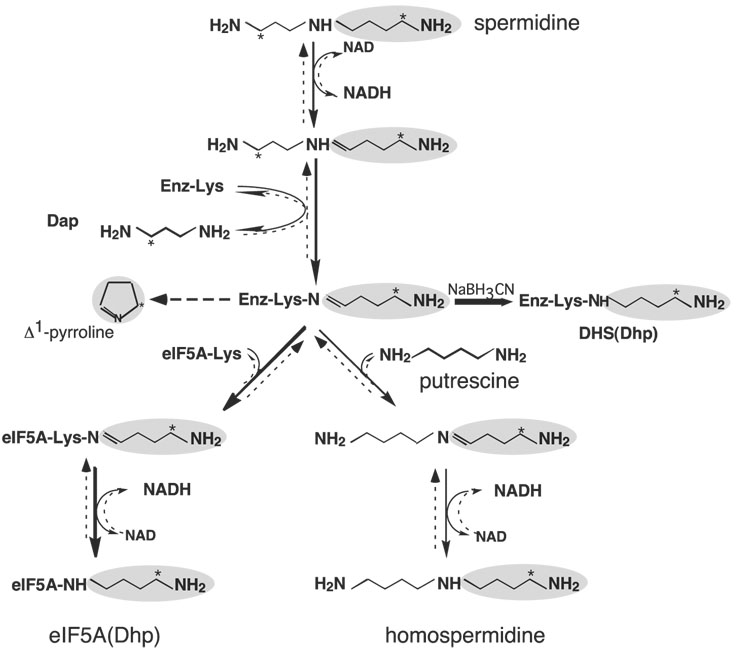
Fig. 3. Mechanism of the deoxyhypusine synthase reaction.
The pathways from spermidine leading to deoxyhypusine synthesis and to homospermidine synthesis are indicated by solid arrows. The reversal pathways from eIF5A(Dhp) back to spermidine and from homospermidine to spermidine are indicated by broken arrows. The 4-aminobutyl moiety is shaded and the position of [3H] derived from [1,8-8H]spermidine is indicated by *. In the absence of acceptor, the 4-aminobutyl moiety of the enzyme-imine intermediate is cyclized to form Δ1-pyrroline. The reduction of the transient enzymeimine intermediate to a stable adduct by NaCNBH3 is indicated by a bold arrow. Abbreviations are: Dap, 1,3-diaminopropane; DHS(Dhp): deoxyhypusine-containing deoxyhypusine synthase. Modified from Ref. 45.