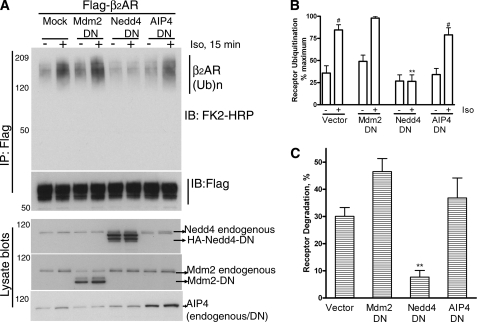
FIGURE 2.
A dominant negative Nedd4 inhibits isoproterenol-stimulated β2AR ubiquitination and degradation. A, 293β2AR were transfected with vector or catalytically inactive Nedd4, Mdm2, or AIP4, and receptors were immunoprecipitated under unstimulated or agonist-stimulated conditions and probed for ubiquitination as in Fig. 1A. Receptor amounts in each sample were determined by reprobing the blot with a M2 Flag antibody (second panel from top). The expression levels of Nedd4-DN, Mdm2-DN, and AIP4-DN are shown in the respective lysate blots. In each case, the band corresponding to endogenous Nedd4, Mdm2, and AIP4 is also indicated. B, quantification of ubiquitination signals from three independent experiments is plotted as a bar graph, where maximum signal is set as 100%. #, p < 0.001 compared with the respective nonstimulated samples; **, <0.01, Nedd4 stimulated versus all other stimulated samples, ANOVA, Bonferroni post-test. C, 293β2AR were transfected with vector, Nedd4-DN, Mdm2-DN, or AIP4-DN and stimulated with isoproterenol for 24 h or not. The receptor levels were determined by 125I-CYP binding. The percent decrease in receptor amounts compared with levels under no stimulation was calculated and plotted as a bar graph, which summarizes data from 3–5 experiments. Data were analyzed by one-way ANOVA. **, p < 0.01.