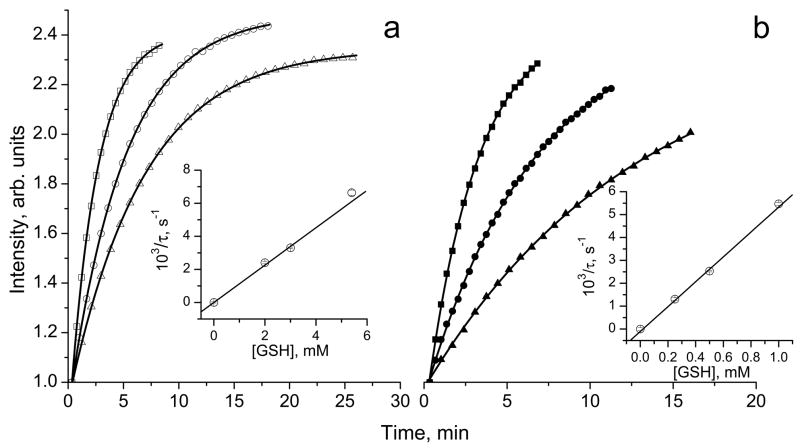
Fig. 2.
The kinetics of the increase of amplitude of the monoradical component, Im, of the EPR spectrum of 10 μM solution in 0.1 M Na-phosphate buffer, pH 7.4, 1 mM DTPA, measured at (a) 23°C and (b) 37°C after addition of various concentrations of GSH: (△) 2 mM, (○) 3 mM, (□) 5.4 mM and (▲) 0.25 mM, (●) 0.5 mM and (■) 1 mM. Lines represent the best fit of the experimental kinetics to the monoexponents, , yielding the values of the characteristic time constant of the exponential growth, τ. Inserts: the dependences of inverse characteristic time constant of the exponential kinetics, 1/τ, on GSH concentration at (a) 23°C and (b) 37°C. The linear regression provides the values of the observed rate constant of the reaction of thiol-disulfide exchange between GSH and (1/τ = kobs×[GSH]) at pH 7.4 yielding kobs(pH 7.4, 23 °C) = 1.13±0.01 M−1 s−1 and kobs(pH 7.4, 37 °C) = 5.4±0.1 M−1 s−1.