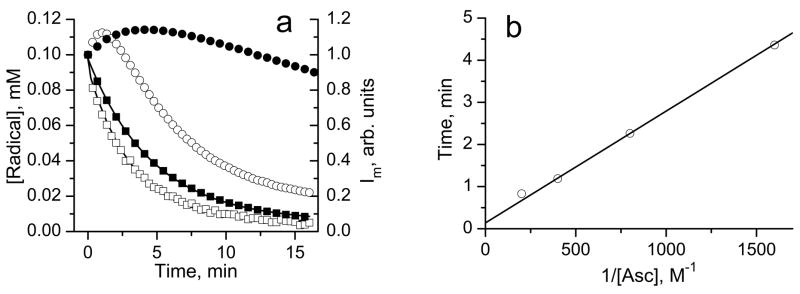
Fig. 4.
Fig. 4a. The kinetics of integral intensities decay of the EPR spectra of 50 μM biradical (□), concentration is multiplied by factor 2 to account for two monoradical subunits) and 100 μM monoradicals, and (■), solutions in 0.1 M Na-phosphate buffer, pH 7.4, 1 mM DTPA, after addition of 2.5 mM ascorbic acid. The solution of the monoradicals was obtained by mixing 0.5 mM of with 1.5 mM of GSH in 10 mM aqueous NaOH and incubating for about 10 min to complete the biradical disulfide splitting monitored by EPR, then a 10 μl aliquot was diluted in 0.1 M Na-phosphate buffer, pH 7.4, 1 mM DTPA, and ascorbate was added to initiate the kinetics. Lines are the best exponential fits yielding the bimolecular rate constants for reduction of the biradical, (1.7±0.2) M−1 s−1, and monoradicals, (1.5±0.2) M−1 s−1. The bell-shaped curves represent the kinetics of the low-field EPR peak intensity, Im, of the solution of the biradical in 0.1 M Na-phosphate buffer, pH 7.4, 1 mM DTPA, after addition of (○) 2.5 mM and (●) 0.625 mM ascorbic acid.
Fig 4b. The dependence of the time point, tmax, corresponding to the maximum of the bell-shaped kinetics of the low-field EPR peak intensity of the solution of the biradical in 0.1 M Na-phosphate buffer, pH 7.4, 1 mM DTPA, on the inverse concentration of reducing agent, ascorbate. The linear regression yields the value of kr equal to (2.2±0.2) M−1 s−1.