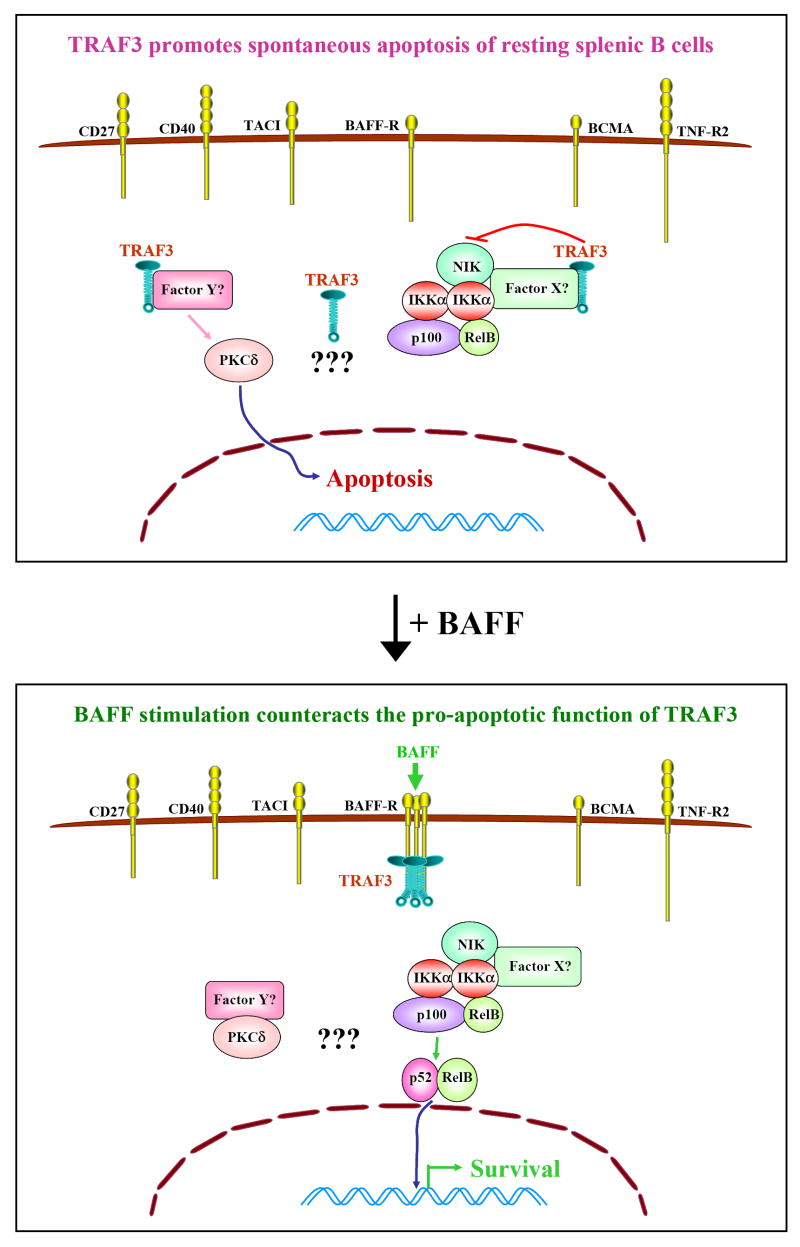
Figure 1. Constitutive function of TRAF3 in mature B cells.
Although six TRAF3-binding receptors are expressed on resting splenic B cells, the predominant function of TRAF3 not associated with receptors is to promote spontaneous apoptosis. In the absence of receptor engagement, TRAF3 is evenly distributed in the cytoplasm of B cells. However, TRAF3 may bind to the kinase NIK directly or indirectly through an unknown factor X, and thus inhib p100 processing. Meanwhile, TRAF3 may bind to another unknown Factor Y (or a multi-protein complex Y), and thus prevent factor Y from binding to PKCδ, allowing PKCδ to enter the nucleus. This explains why, in the absence of stimulation, mouse splenic B cells die by apoptosis. It is also possible that yet unknown pathways may be involved. Upon BAFF signaling, BAFF-R is trimerized or multimerized, and TRAF3 is recruited to the BAFF-R signaling complex in membrane rafts. Thus, BAFF signaling functions to remove TRAF3 from its cytoplasmic binding partners. Factor Y can then bind to PKCδ, and sequester it in the cytosol. Meanwhile, NIK can activate IKKα to process p100 into p52, allowing p52/RelB dimers to move into the nucleus and activate transcription of antiapoptotic proteins, leading to B cell survival.