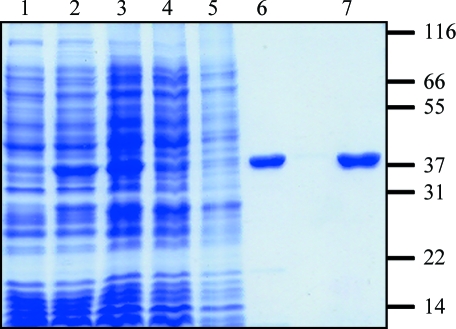
Figure 1.
Purification of recombinant SaBPL-H6. The purification strategy was monitored by SDS–PAGE on a 12% polyacrylamide gel. The His6-tagged protein migrated at a relative mobility of ∼38 kDa. Lane 1 contains crude cell lysate before recombinant protein expression, lane 2 contains crude lysate after induction with 1 mM IPTG and lane 3 contains 50 µg of the soluble fraction after cell lysis. SaBPL-H6 was then purified by IMAC chromatography (lane 4, 50 µg unbound protein; lane 5, 50 µg of the wash fraction; lane 6, 5 µg eluted protein) and S-Sepharose ion-exchange chromoatography (lane 7, 5 µg of SaBPL-H6 from pooled fractions containing BPL activity). Molecular-weight standards (labelled in kDa) are shown on the right.