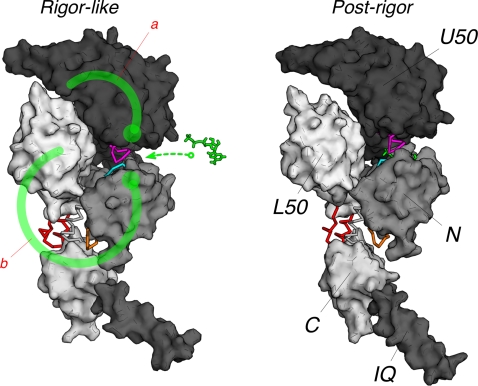
Figure 12. Molecular mechanism of the rigor-like to post-rigor transition as described by the NMSM pathway.
The molecular surface of the myosin subdomains is shown in tones of grey; the nucleotide is in green. Key residues at the subdomain interfaces that are responsible for the coupling between the myosin subdomains are depicted in cyan, magenta, orange, and red; they correspond to the P-loop, switch I, loop 76–81, and the relay helix, respectively. These allosteric connectors couple the local changes due to ATP binding to the more global motions of the myosin molecule. The ATP-binding signal is transmitted to the U50/L50 cleft through two distinct communication pathways (shown as heavy green lines; the large dot indicates the approximate origin of the signal). Path a involves the U50 subdomain and is consistent with the interpretation of three-dimensional electron cryo-microscopy reconstructions by Holmes et al. [60]. Path b involves the N-terminal subdomain, the converter and the L50 subdomain. In the latter, the transmission of the ATP binding signal is the consequence of the coordinated movement of the three subdomains described by the NMSM path (see Video S1). The allosteric communication results in the opening of the U50/L50 cleft and the uncoupling of the converter from the motor head.