FIGURE 3.
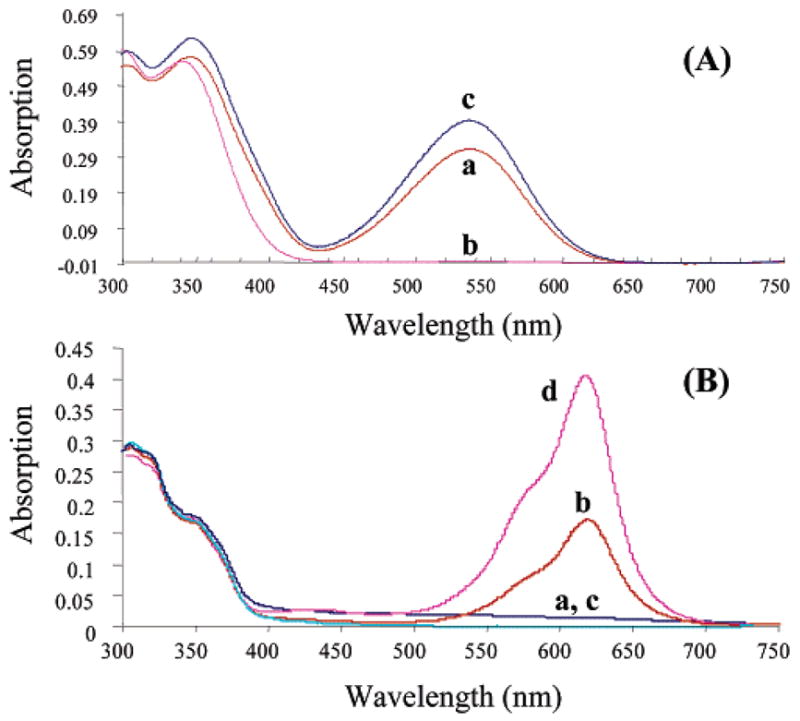
Absorption spectra of compound 13 (A) and 17 (B) in propylene glycol at 0 °C. (A) The ability to optically control the MC to SP states of spirobenzopyrans is shown in the spectrum of a 10 μM solution of 13 following a 10 s irradiation using 365 nm light (a), followed by 10 s of 546 nm light (b), and 10 s of 365 nm light (c). (B) The ability to optically control the MC to SP states of spironaphthozaxine (17) is shown in the spectrum of a 10 μM solution of 17 following a 10 s irradiation using 546 nm light (a), followed by 10 s of 365 nm light (b), 10 s of 546 nm light (c) and finally 10 s of 365 nm light (d). The difference in the absorption value for the MC state between switch cycles observed in Figure 3A is caused by slight differences in the method of illumination, while in Figure 3B, the different MC absorption values result from the rapid, thermally driven MC to SP transition (time constant of 6 s) and simply reflect a difference in the period between the UV illumination of the SP state and the absorption measurement.
