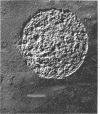
Abstract
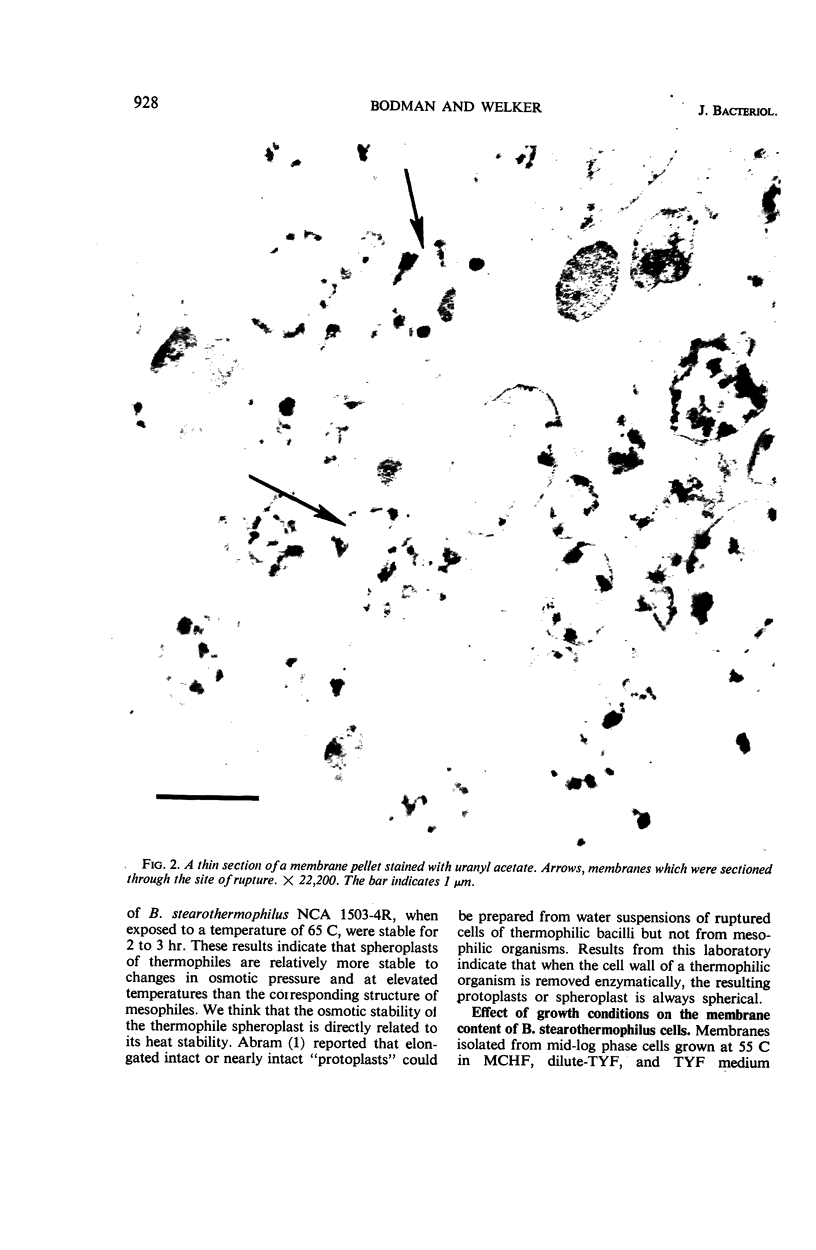
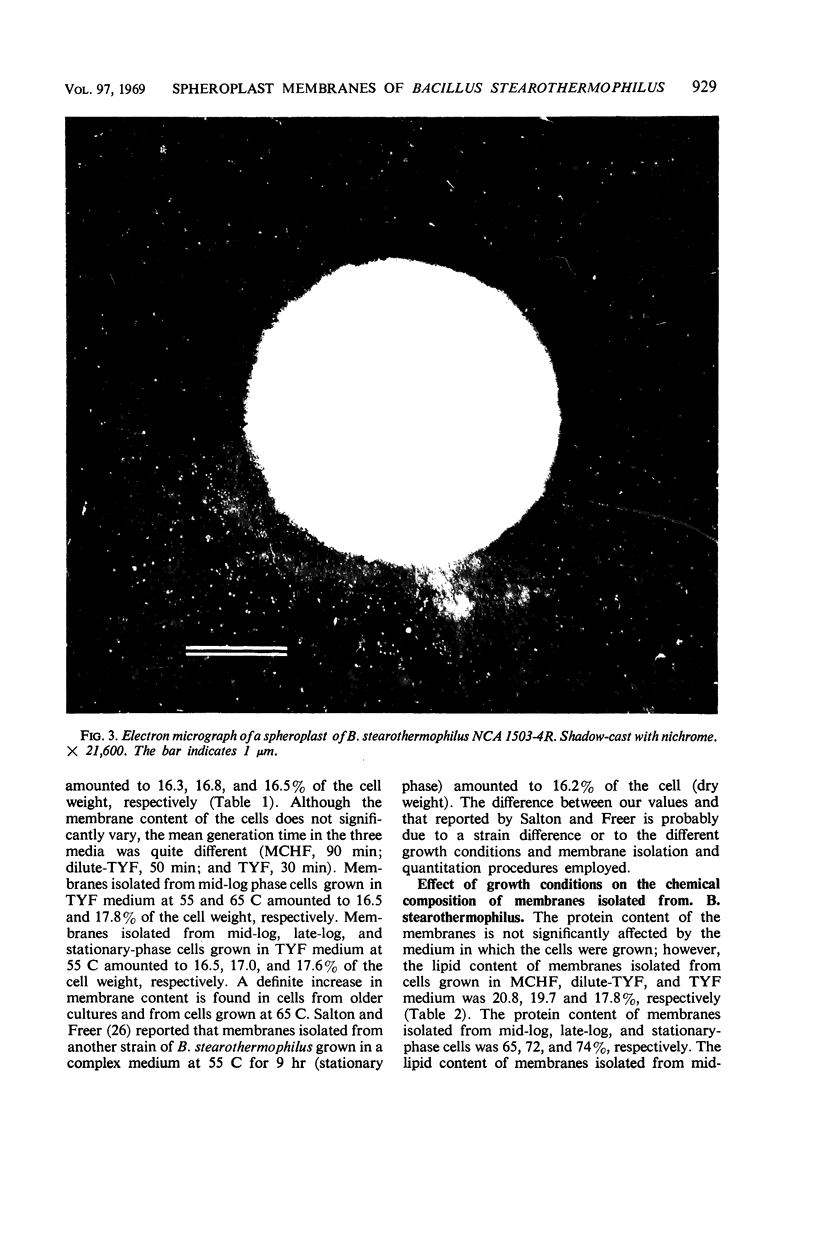
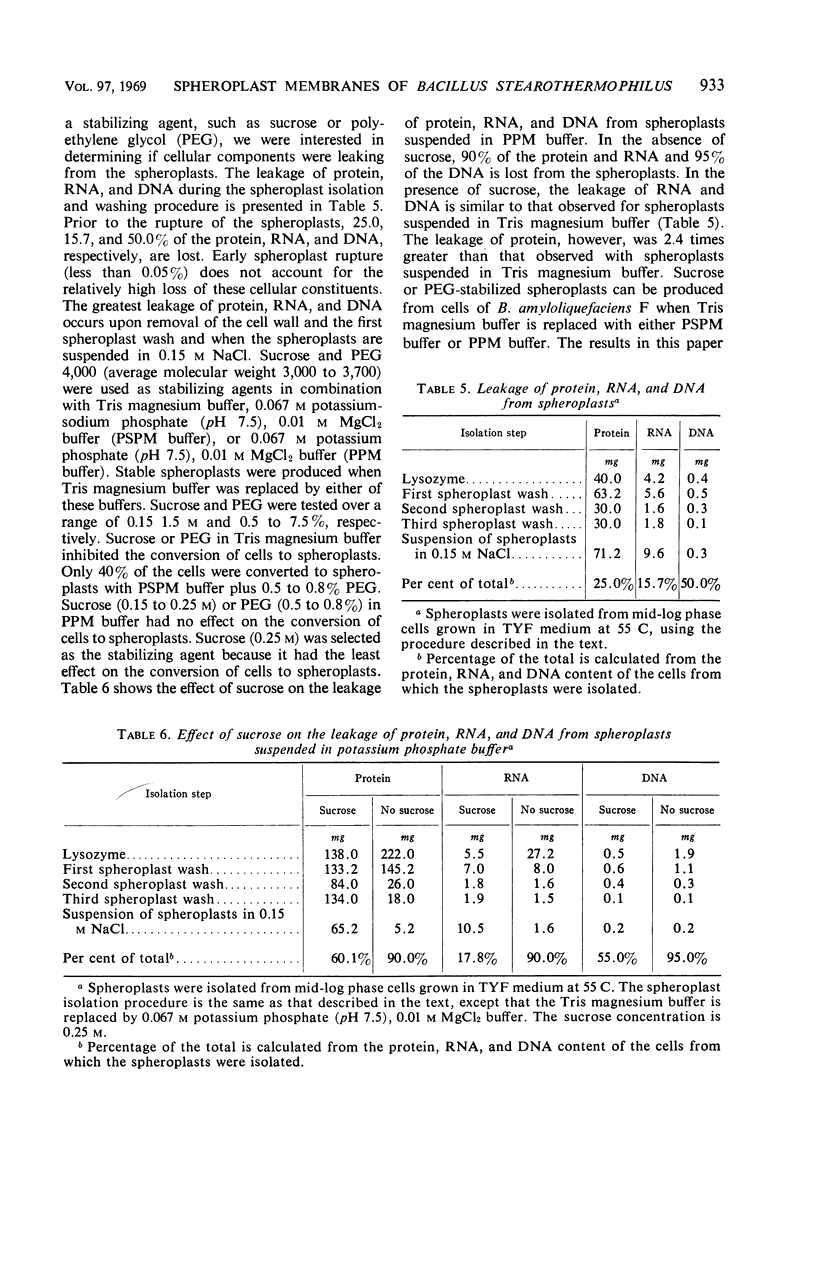
Spheroplasts were prepared by lysozyme digestion of the cell wall and ruptured by suspension in 0.15 m NaCl, followed by centrifugation at 30,900 × g for 35 min, and by a final suspension in 0.05 m NaCl for 12 to 16 hr at 5 C. The membrane ghosts were washed four times in tris(hydroxylmethyl)aminomethane (Tris) magnesium buffer and once in distilled water. The intact membranes resembled empty sacs with narrow slits in which the cytoplasm was extruded. A 92% recovery of cell membrane was obtained with all membrane preparations. The spheroplasts do not require a stabilizing medium to keep them from rupturing, and they are stable for 2 to 3 hr when exposed to a temperature of 65 C. The membrane content of the cell increases with age of culture (mid-log, 16.5%; late-log, 17.0%; and stationary, 17.6%) and temperature of growth (55 C, 16.5%; and 65 C, 17.8%), and it is unaffected by composition of the growth medium. The ratio of the protein to lipid content of the membrane increases with the complexity of the medium, age of culture (mid-log, 3.65; late-log, 3.91; and stationary, 4.15), and temperature of growth (55 C, 3.65; and 65 C, 5.22). The ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) content of the membranes was 9.0 to 13.7% and 0.3 to 0.8%, respectively. Reducing sugar (determined as glucose) amounts to 0.9 to 1.0% of the membrane weight and did not significantly vary for the different membrane preparations. Medium composition, age of culture, and temperature of growth have no significant effect on the amount of each amino acid in the membrane. Aspartic acid, glutamic acid, alanine, leucine, and lysine are present in the greatest amount and represent 12.9 to 14.1%, 10.4 to 11.3%, 9.6 to 10.3%, 7.7 to 8.8%, and 7.6 to 8.5% of the membrane peptide, respectively. Prior to the rupture of the spheroplasts, 25.0, 15.7, and 50.0% of the protein, RNA, and DNA, respectively, is lost. In potassium phosphate-magnesium buffer without sucrose, 90% of the protein and RNA and 95% of the DNA is lost from the spheroplasts. In the presence of sucrose, the leakage of RNA and DNA is similar to that observed for spheroplasts suspended in Tris magnesium buffer; however, the leakage of protein is 2.4 times greater.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAM D. ELECTRON MICROSCOPE OBSERVATIONS ON INTACT CELLS, PROTOPLASTS, AND THE CYTOPLASMIC MEMBRANE OF BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1965 Mar;89:855–873. doi: 10.1128/jb.89.3.855-873.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLEN M. B. The thermophilic aerobic sporeforming bacteria. Bacteriol Rev. 1953 Jun;17(2):125–173. doi: 10.1128/br.17.2.125-173.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D. Life at high temperatures. Evolutionary, ecological, and biochemical significance of organisms living in hot springs is discussed. Science. 1967 Nov;158(3804):1012–1019. doi: 10.1126/science.158.3804.1012. [DOI] [PubMed] [Google Scholar]
- Campbell L. L., Pace B. Physiology of growth at high temperatures. J Appl Bacteriol. 1968 Mar;31(1):24–35. doi: 10.1111/j.1365-2672.1968.tb00338.x. [DOI] [PubMed] [Google Scholar]
- Cho K. Y., Salton M. R. Fatty acid composition of bacterial membrane and wall lipids. Biochim Biophys Acta. 1966 Feb 1;116(1):73–79. doi: 10.1016/0005-2760(66)90093-2. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Farrell J., Rose A. Temperature effects on microorganisms. Annu Rev Microbiol. 1967;21:101–120. doi: 10.1146/annurev.mi.21.100167.000533. [DOI] [PubMed] [Google Scholar]
- Friedman S. M. Protein-synthesizing machinery of thermophilic bacteria. Bacteriol Rev. 1968 Mar;32(1):27–38. doi: 10.1128/br.32.1.27-38.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILBY A. R., FEW A. V., McQUILLEN K. The chemical composition of the protoplast membrane of Micrococcus lysodeikticus. Biochim Biophys Acta. 1958 Jul;29(1):21–29. doi: 10.1016/0006-3002(58)90141-0. [DOI] [PubMed] [Google Scholar]
- Gaughran E. R. THE THERMOPHILIC MICROORGANISMS. Bacteriol Rev. 1947 Sep;11(3):189–225. doi: 10.1128/br.11.3.189-225.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B. K., Carroll K. K. Isolation, composition, and structure of membrane of Listeria monocytogenes. J Bacteriol. 1968 Feb;95(2):688–699. doi: 10.1128/jb.95.2.688-699.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOFFLER H. Protoplasmic differences between mesophiles and thermophiles. Bacteriol Rev. 1957 Dec;21(4):227–240. doi: 10.1128/br.21.4.227-240.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONG S. K., WILLIAMS O. B. Lipids of Bacillus stearothermophilus. J Bacteriol. 1960 May;79:629–637. doi: 10.1128/jb.79.5.629-637.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Autolytic release and osmotic properties of protoplasts from Staphylococcus aureus. J Gen Microbiol. 1957 Feb;16(1):184–194. doi: 10.1099/00221287-16-1-184. [DOI] [PubMed] [Google Scholar]
- SCHLESSINGER D., MARCHESTI V. T., KWAN B. C. BINDING OF RIBOSOMES TO CYTOPLASMIC RETICULUM OF BACILLUS MEGATERIUM. J Bacteriol. 1965 Aug;90:456–466. doi: 10.1128/jb.90.2.456-466.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOCKMAN G. D., KOLB J. J., BAKAY B., CONOVER M. J., TOENNIES G. Protoplast membrane of Streptococcus faecalis. J Bacteriol. 1963 Jan;85:168–176. doi: 10.1128/jb.85.1.168-176.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton M. R., Freer J. H. Composition of the membranes isolated from several Gram-positive bacteria. Biochim Biophys Acta. 1965 Oct 18;107(3):531–538. doi: 10.1016/0304-4165(65)90197-2. [DOI] [PubMed] [Google Scholar]
- Salton M. R. Structure and function of bacterial cell membranes. Annu Rev Microbiol. 1967;21:417–442. doi: 10.1146/annurev.mi.21.100167.002221. [DOI] [PubMed] [Google Scholar]
- Scholes P. B., Smith L. The isolation and properties of the cytoplasmic membrane of Micrococcus denitrificans. Biochim Biophys Acta. 1968 Feb 12;153(2):350–362. doi: 10.1016/0005-2728(68)90080-7. [DOI] [PubMed] [Google Scholar]
- Sutow A. B., Welker N. E. Chemical composition of the cell walls of Bacillus stearothermophilus. J Bacteriol. 1967 Apr;93(4):1452–1457. doi: 10.1128/jb.93.4.1452-1457.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C., BERGSTROM L. The chemical nature of the cytoplasmic membrane and cell wall of Bacillus megaterium, strain M. Biochim Biophys Acta. 1958 Nov;30(2):340–351. doi: 10.1016/0006-3002(58)90059-3. [DOI] [PubMed] [Google Scholar]
- WEIBULL C. The isolation of protoplasts from Bacillus megaterium by controlled treatment with lysozyme. J Bacteriol. 1953 Dec;66(6):688–695. doi: 10.1128/jb.66.6.688-695.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELKER N. E., CAMPBELL L. L. EFFECT OF CARBON SOURCES ON FORMATION OF ALPHA-AMYLASE BY BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1963 Oct;86:681–686. doi: 10.1128/jb.86.4.681-686.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELKER N. E., CAMPBELL L. L. INDUCTION AND PROPERTIES OF A TEMPERATURE BACTERIOPHAGE FROM BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1965 Jan;89:175–184. doi: 10.1128/jb.89.1.175-184.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. B., Perkins H. R. The chemical composition of the membranes of protoplasts and L-forms of Staphylococcus aureus. Biochem J. 1968 Jan;106(2):391–400. doi: 10.1042/bj1060391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker N. E., Campbell L. L. Unrelatedness of Bacillus amyloliquefaciens and Bacillus subtilis. J Bacteriol. 1967 Oct;94(4):1124–1130. doi: 10.1128/jb.94.4.1124-1130.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker N. E. Purification and properties of a thermophilic bacteriophage lytic enzyme. J Virol. 1967 Jun;1(3):617–625. doi: 10.1128/jvi.1.3.617-625.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]