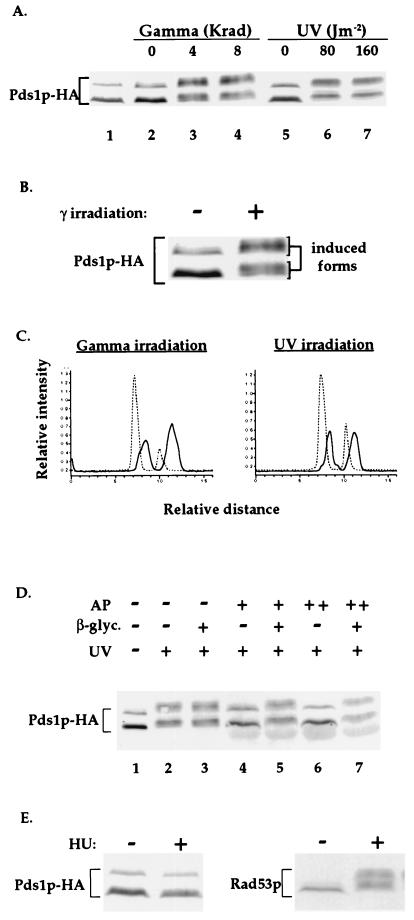
Figure 1.
Pds1p-HA is phosphorylated in the presence of DNA damage but not when DNA replication is inhibited. (A) Wild-type OCF1522 cells grown at 30°C were arrested in mitosis with nocodazole (lanes 2–7) and exposed to either UV- or γ-radiation at the indicated doses. Protein samples were prepared 20 min after irradiation and processed for Western blot analysis. The sample from the cycling cells (lane 1) was prepared from cells of the same culture taken prior to the addition of nocodazole. The protein bands corresponding to Pds1p-HA are indicated. Occasionally, a fast-migrating HA-cross-reacting band appears (data not shown). (B) An enlargement of the Pds1-HA bands from lanes 2 and 3 of A. (C) Densitometry scan of lanes 2, 4 (Left), 5, and 7 (Right) of A. The dashed and solid lines are of Pds1p-HA from nonirradiated and irradiated cells, respectively. Scans were preformed from bottom to top. (D) Protein extracts were prepared from nocodazole-arrested wild-type OCF1522 cells that were UV-irradiated at 80 J/m2 as described above. Reactions were carried out in the absence (−) or presence of calf intestine alkaline phosphatase (AP; +, 40 units per reaction; ++, 80 units per reaction) and in the absence (−) or presence (+) of the alkaline phosphatase inhibitor β-glycerophosphate (β-glyc.). (E) Wild-type OCF1522 cells grown at 30°C were not treated (−) or treated with 0.1 M HU for 2 h, after which protein extracts were prepared and examined by Western blot analysis for Pds1p-HA (Left) and Rad53p (Right). Identical results were obtained when cells were arrested with 0.2 M HU (data not shown). The S phase arrest after the HU treatment was verified by flow cytometry analysis (data not shown).