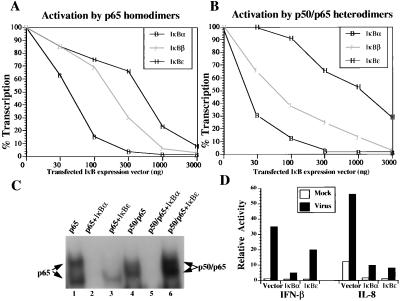
Figure 3.
IκBɛ preferentially inhibits the p65 homodimeric form of NF-κB in vivo. (A) Mouse P19 cells were cotransfected with the PRDII-CAT reporter plasmid (200 ng) and a constant amount of p65 expression vector (100 ng) along with the indicated amounts of expression plasmids for IκBα, IκBβ, and IκBɛ. 100% transcription corresponds to the level of activation (67-fold) obtained in the absence of IκB plasmids. Shown is one of three independent experiments. The variability from experiment to experiment was less than 15%. (B) Same as in A, but the activator was an equimolar mixture of p50 and p65 expression vectors (100 ng). 100% corresponds to a 47-fold induction of transcription in the absence of IκB expression vectors. (C) Shown is an EMSA experiment using the PRDII oligonucleotide as a probe along with extracts prepared from COS cells transfected with the expression vectors indicated on the top of the gel. The identity of the complexes was verified by antibody supershift experiments (not shown). Transfection of p65 results in the formation of two complexes because of proteolytic cleavage during extract preparation. (D) Mouse 3T3 cells were cotransfected with the −110IFN-β-CAT or IL-8 luciferase (4 μg) along with IκBα or IκBɛ expression vectors (16 μg). Thirty-six hours after transfection the cells were infected with Sendai virus for 8 h, and the CAT and luciferase activities were determined.