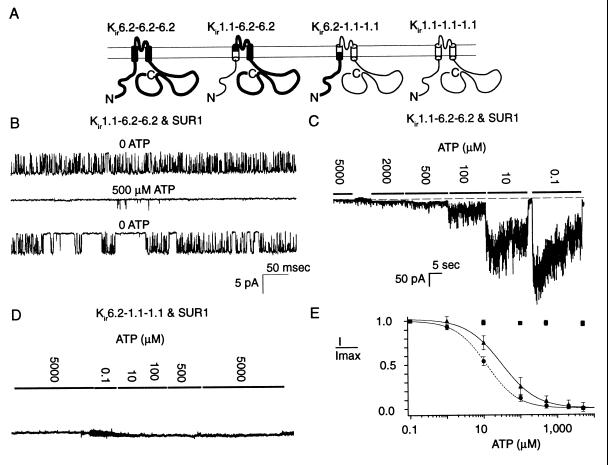
Figure 2.
The N-terminal cytoplasmic segment of Kir6.2 is unlikely to be a major determinant of the ATP-dependent inhibition gating. Each construct was coexpressed with SUR1 and studied at −80 mV. (A) Parental and chimeric Kir constructs in which the N-terminal cytoplasmic segments are swapped. (B) Single channel records showing strong ATP sensitivity of channels formed from Kir1.1–6.2–6.2 and SUR1. (C) Corresponding macroscopic currents. (D) Macroscopic currents (≈730 pA) through channels formed from Kir6.2–1.1–1.1 show no ATP sensitivity. (E) Hill plots of ATP sensitivity of the chimeric constructs: •, wild-type Kir6.2 channels, Ki = 12.3 ± 3.5 μM and αH = 1.03 ± 0.10 (n = 8; taken from Fig. 1B); ▴, channels formed from Kir1.1–6.2–6.2, Ki = 30.0 ± 5.4 μM and αH = 1.0 ± 0.2 (n = 4); and ■, Kir6.2–1.1–1.1 (insensitive to ATP; n = 5).