Abstract
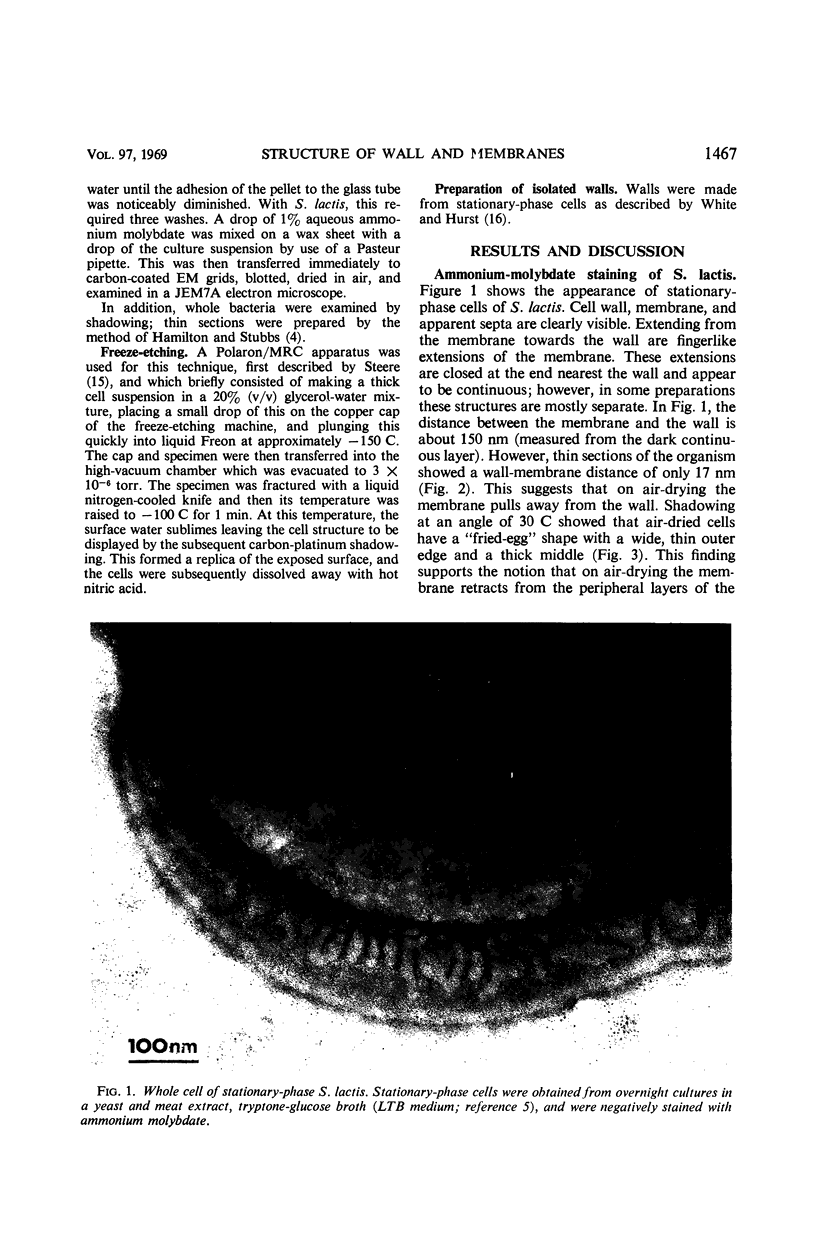
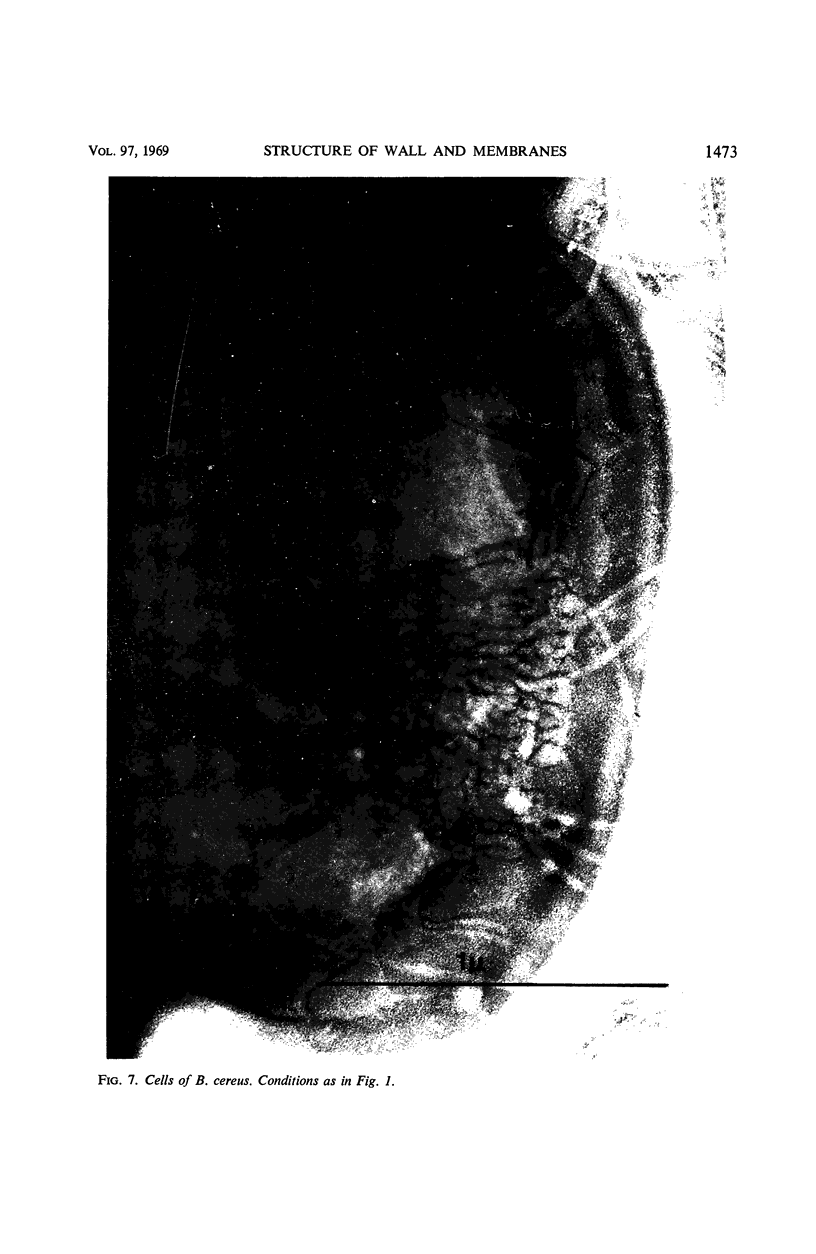
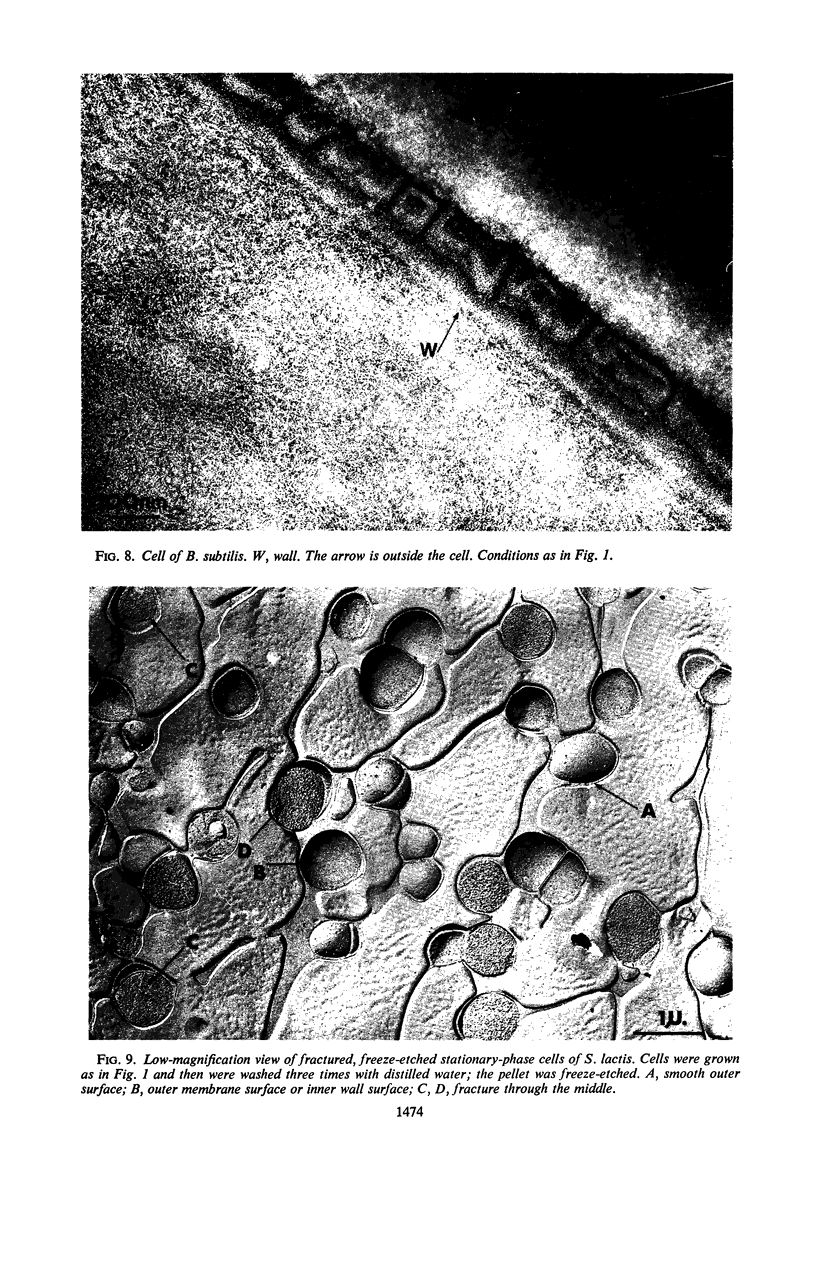
Thin sections of stationary-phase Streptococcus lactis cells showed that the wall and membrane are 20 and 7 nm thick, respectively. Whole cells were examined by negative staining with ammonium molybdate and by shadowing. On air-drying of whole cells, the membrane pulled away from the wall revealing adhesions between these organelles. Adhesions could not be seen after subculture of the stationary-phase cells into complex media or into solutions containing glucose, KCl, and CaCl2 in tris(hydroxymethyl)aminomethane buffer. The adhesions were also observed in stationary-phase cells of other gram-positive bacteria. Fractured freeze-etched cells of S. lactis had a smooth outside surface, but the inside of the wall (or outside of the membrane) had a regular structure, repeating at 10 nm, which could correspond to the adhesions observed in the negatively stained air-dried cells. Freeze-etching also revealed holes in the outside wall which had the shape of inverted truncated cones. The outside diameter of the cone was 60 nm, and the diameter on the inside surface of the wall was 20 nm. The membrane had upstanding plugs, 20 nm in diameter, which could fill the holes in the wall.
Full text
PDF





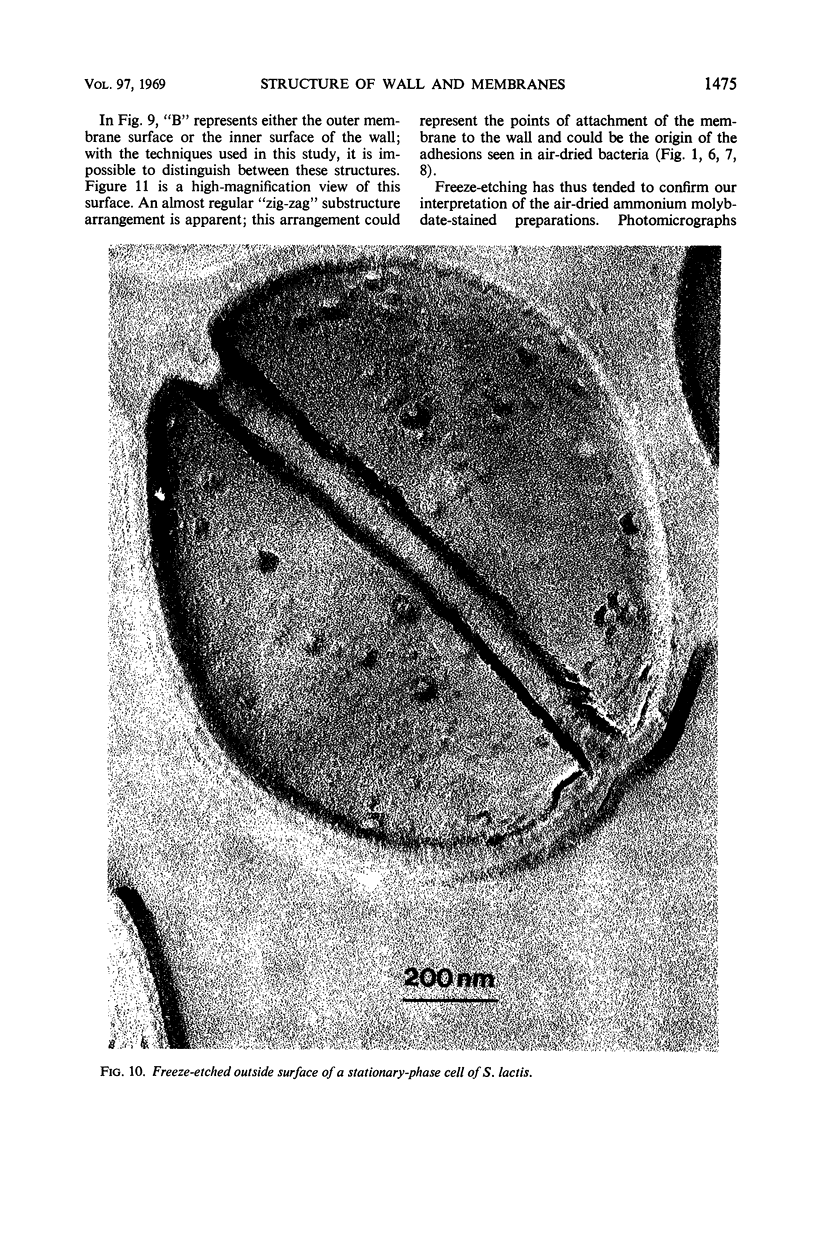
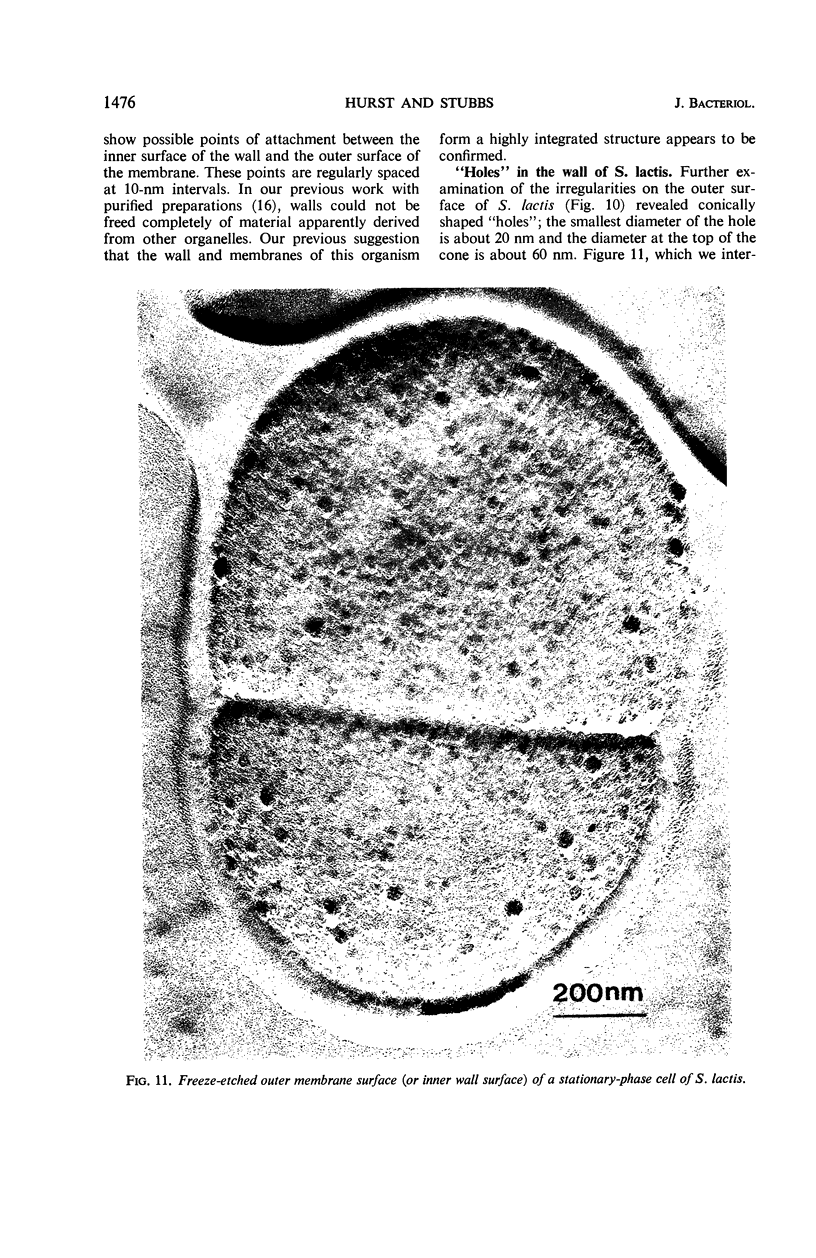
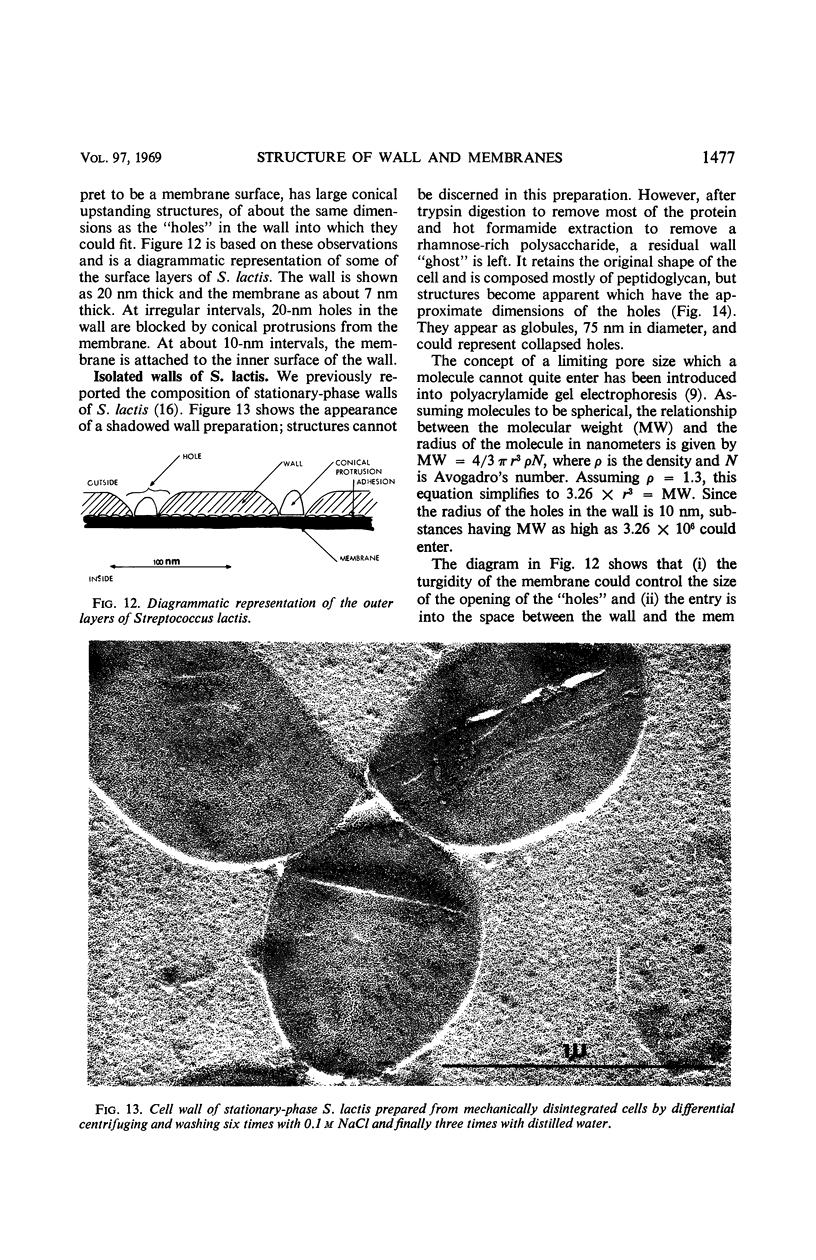
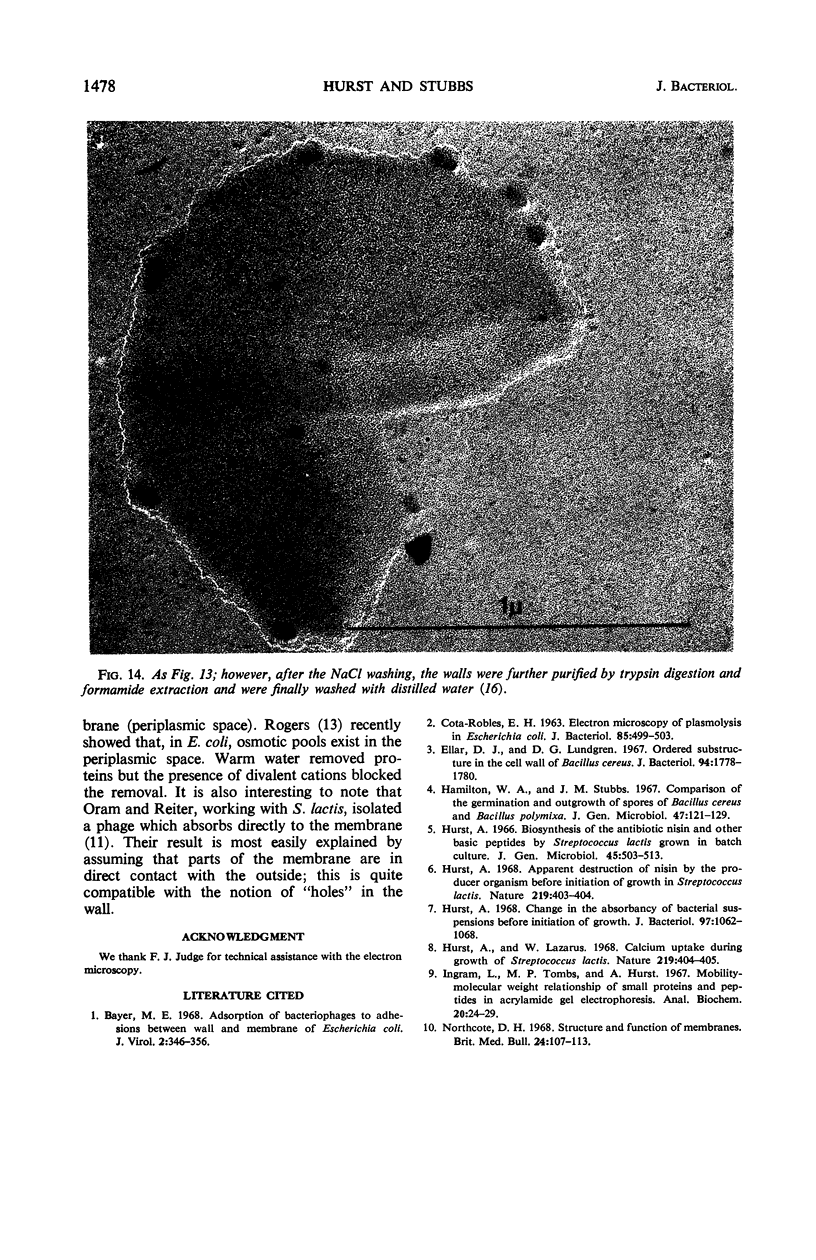

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E. Adsorption of bacteriophages to adhesions between wall and membrane of Escherichia coli. J Virol. 1968 Apr;2(4):346–356. doi: 10.1128/jvi.2.4.346-356.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTA-ROBLES E. H. ELECTRON MICROSCOPY OF PLASMOLYSIS IN ESCHERICHIA COLI. J Bacteriol. 1963 Mar;85:499–503. doi: 10.1128/jb.85.3.499-503.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellar D. J., Lundgren D. G. Orded substructure in the cell wall of Bacillus cereus. J Bacteriol. 1967 Nov;94(5):1778–1780. doi: 10.1128/jb.94.5.1778-1780.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W. A., Stubbs J. M. Comparison of the germination and outgrowth of spores of Bacillus cereus and Bacillus polymyxa. J Gen Microbiol. 1967 Apr;47(1):121–129. doi: 10.1099/00221287-47-1-121. [DOI] [PubMed] [Google Scholar]
- Hurst A. Apparent destruction of nisin by the producer organism before initiation of growth in Streptococcus lactis. Nature. 1968 Jul 27;219(5152):403–404. doi: 10.1038/219403b0. [DOI] [PubMed] [Google Scholar]
- Hurst A. Change in the absorbancy of bacterial suspensions before initiation of growth. J Bacteriol. 1969 Mar;97(3):1062–1068. doi: 10.1128/jb.97.3.1062-1068.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst A., Lazarus W. Calcium uptake during growth of Streptococcus lactis. Nature. 1968 Jul 27;219(5152):404–405. doi: 10.1038/219404a0. [DOI] [PubMed] [Google Scholar]
- Ingram L., Tombs M. P., Hurst A. Mobility-molecular weight relationships of small proteins and peptides in acrylamide-gel electrophoresis. Anal Biochem. 1967 Jul;20(1):24–29. doi: 10.1016/0003-2697(67)90260-6. [DOI] [PubMed] [Google Scholar]
- Northcote D. H. Structure and function of plant-cell membranes. Br Med Bull. 1968 May;24(2):107–112. doi: 10.1093/oxfordjournals.bmb.a070609. [DOI] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. The adsorption of phage to group N streptococci. The specificity of adsorption and the location of phage receptor substances in cell-wall and plasma-membrane fractions. J Gen Virol. 1968 Jul;3(1):103–119. doi: 10.1099/0022-1317-3-1-103. [DOI] [PubMed] [Google Scholar]
- Remsen C. C. Fine structure of the mesosome and nucleoid in frozen-etched Bacillus subtilis. Arch Mikrobiol. 1968;61(1):40–47. doi: 10.1007/BF00704290. [DOI] [PubMed] [Google Scholar]
- Rogers D. Osmotic pools in Escherichia coli. Science. 1968 Feb 2;159(3814):531–532. doi: 10.1126/science.159.3814.531. [DOI] [PubMed] [Google Scholar]
- Ryter A. Association of the nucleus and the membrane of bacteria: a morphological study. Bacteriol Rev. 1968 Mar;32(1):39–54. doi: 10.1128/br.32.1.39-54.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEERE R. L. Electron microscopy of structural detail in frozen biological specimens. J Biophys Biochem Cytol. 1957 Jan 25;3(1):45–60. doi: 10.1083/jcb.3.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J., Hurst A. The location of nisin in the producer organism, Streptococcus lactis. J Gen Microbiol. 1968 Sep;53(2):171–179. doi: 10.1099/00221287-53-2-171. [DOI] [PubMed] [Google Scholar]