Figure 9. Modulation of Wnt signalling by Notch in Drosophila (see text for details of interactions).
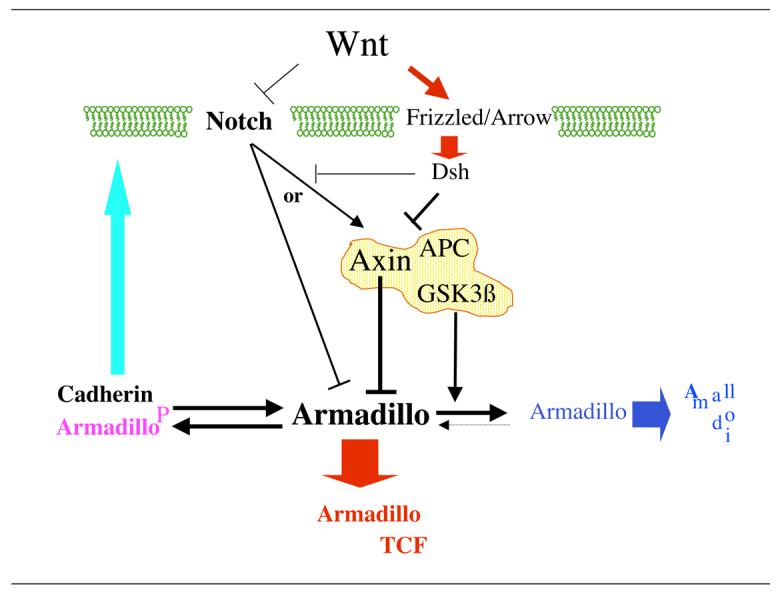
In the steady state, Armadillo exists in a number of molecularly distinct pools which appear to be in equilibrium. Armadillo associates readily with Cadherin and participated in the dynamics of adherens junctions. On the other hand, its association with a complex, which includes Axin and APC, leads to its phosphorylation on the N terminus by GSK3ß (Shaggy) and the delivery of the phosphorylated form to the proteasome where it is degraded. In addition to serving as a scaffold for GSK3ß mediated phosphorylation of Armadillo, Axin can prevent the formation of its active complex with TCF in a GSK3ß independent manner. Our results indicate that Notch modulates the activity and amounts of hypophosphorylated Armadillo either by targeting the GSK3ß independent activity of Axin or via an independent mechanism. Wnt signalling through a Frizzled/Arrow (LRP5/6) heterodimer activates Dishevelled which inactivates the complex, destroying Axin and inhibiting the N terminal phosphorylation of Arm. Wnt has been shown to bind to the extracellular domain of Notch (Brennan et al., 1999a; Wesley, 1999) and Dishevelled to the intracellular domain ((Axelrod et al., 1996)and this work) and this binding is likely to be responsible for down regulating the modulatory activity of Notch. The net effect of the inactivation of Notch and the Axin based complex results in an efficient accumulation of Armadillo in the nucleus and its interaction with TCF.
