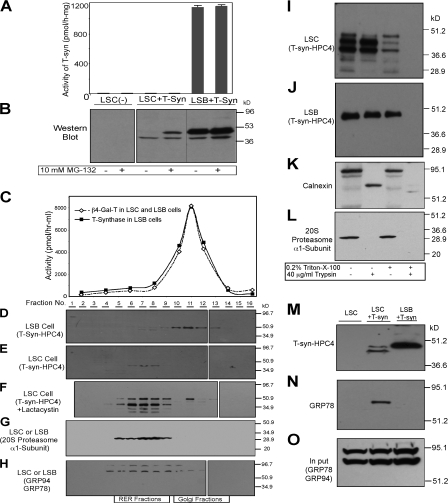
Figure 4.
Characterization of T-synthase expressed in LSC cells containing a dysfunctional Cosmc. (A and B) MG-132 causes accumulation of full-length T-synthase protein in LSC cells but failed to restore its activity. LSC and LSB cells stably expressing T-synthase–HPC4 were treated with 10 μM MG-132 for 12 h and harvested. Cell extract was made and one portion was used for measuring T-synthase activity (A), whereas the other portion was analyzed on SDS-PAGE (30 μg protein/lane) by Western blot with anti-HPC4 (B). Error bars represent ±1 SD from the mean. Black lines indicates that intervening lanes have been spliced out. (C–H) T-synthase expressed in LSC cells resides mainly in heavy membrane fractions (RER), whereas T-synthase from LSB cells is in light membrane fractions (Golgi). LSC and LSB cells as in A and B treated with 10 μM lactacystin were homogenized and cell homogenate was fractionated on a sucrose gradient by ultracentrifugation. The fractions were collected and the activities of β4–Gal-T and T-synthase in fractions were determined (C). The fractions were also analyzed by Western blotting with anti-HPC4 (D–F), anti-20S proteasome α1-subunit (G), and anti-KDEL (GRP78 and GRP94; H). In F, lane 2 represents the combination of fractions 1 and 2. (I–O) T-synthase expressed in LSC cells resides mainly in the lumen of the RER and was associated with GRP78. The cell PNS of LSC and LSB cells as in A and B was digested with 40 μg/ml trypsin in the presence or absence of 0.2% Triton X-100, and T-synthase was analyzed under reducing SDS-PAGE by Western blotting with HPC4 mAb (I and J). As controls, calnexin and 20S proteasome α1-subunit were also analyzed by Western blotting with their respective antibody (K and L). T-synthase was purified from LSC and LSB cells and Western blotted with anti-HPC4 (M) and anti-KDEL (GRP78; N). GRP78 in the cell extract corresponding to one-tenth of the material was detected to confirm comparable starting amounts of material (O).