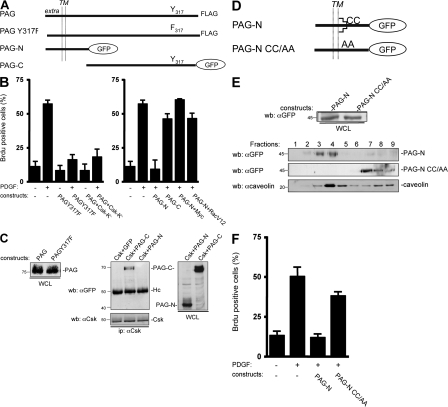
Figure 2.
PAG mitogenic inhibition is independent of Csk but requires PAG palmitoylation sites. (A) Schematic of the PAG constructs used. The extracellular (extra), transmembrane (TM), and cytoplasmic domains are indicated as well as the Csk (Y317) binding site and the GFP fused to PAG sequences. (B) PAG mitogenic inhibition does not require binding to Csk. Quiescent NIH 3T3 cells were transfected with the indicated constructs and stimulated, or not, with PDGF in the presence of BrdU. (C, left) Comparison of the levels of PAG and PAGY317F. (C, right) PAG-N does not bind to Csk unlike PAG-C. Protein levels were assessed by Western blotting with the indicated antibodies from whole cell lysates (WCL) of HEK 293 cells expressing the shown constructs. Csk–PAG complex formation was assessed by coimmunoprecipitation with an anti-Csk antibody. Molecular masses are indicated in kilodaltons. (D) Schematic of the PAG-N constructs used. Palmitoylation sites are indicated. (E) Levels of PAG-N and PAG-NCC/AA in whole cell lysates (top) and in CEF (bottom). HEK 293 cells transfected with the indicated constructs were lysed with Triton X-100 and homogenized with a Dounce, followed by sucrose gradient fractionation. Fractions were then directly analyzed by Western blotting with anti-GFP and anti-caveolin antibodies, as indicated. The respective proteins as well as the fractions' numbers are shown. Molecular masses are indicated in kilodaltons. (F) PAG-N mitogenic inhibition requires the palmitoylation sites. Quiescent NIH 3T3 cells transfected with the indicated constructs were stimulated, or not, with PDGF in the presence of BrdU. Cells were then fixed and processed for immunofluorescence. The percentage of transfected (or infected) cells that incorporated BrdU was calculated as described in Material and methods. Results are expressed as the mean ± SD of three to five independent experiments.