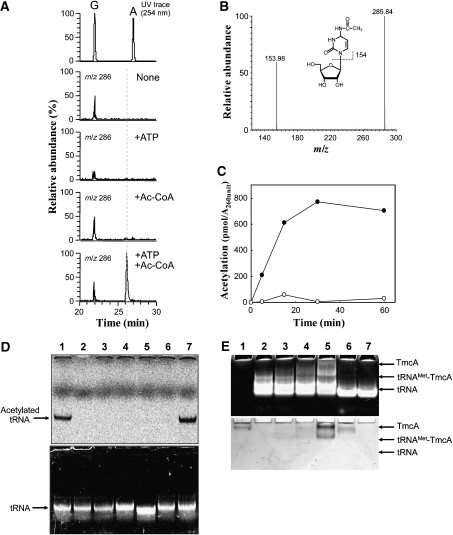
Figure 4.
Reconstitution of ac4C formation in vitro using recombinant TmcA. (A) Mass spectrometric detection of ac4C formation in the unmodified tRNAMet that was transcribed in vitro. After the reaction, the tRNAMet was digested into nucleosides and analysed by LC/MS. Mass chromatograms at m/z 286 detected ac4C, which was reconstituted in vitro in the absence of both ATP and acetyl-CoA (second panel), in the presence of ATP (third panel), in the presence of acetyl-CoA (fourth panel) or in the presence of both ATP and acetyl-CoA (bottom panel). The ac4C appeared only when both substrates were present. (B) Mass spectrum of ac4C that was reconstituted in vitro. (C) In vitro reconstitution of ac4C formation in the presence (filled circle) or absence (open circle) of ATP, which was detected by liquid scintillation counting of 14C-acetyl group. (D) In vitro reconstitution of ac4C formation under various conditions, the radioactivity of which was visualized by an imaging analyser (FLA-7000, Fujifilm). The upper and lower panels show visualized radioactivity and ethidium bromide staining. The reaction was performed in the presence of ATP (lane 1), GTP (lane 7) and ADP (lane 6), in the absence of ATP (lane 3) and TmcA (lane 2). The mutant tRNAMet, EM(C34G), whose C34 was replaced by G34 (lane 4) and tRNAIle2 (lane 5), were employed for ac4C reconstitution instead of tRNAMet. (E) Gel-retardation analysis of tRNAMet with recombinant TmcA. The polyacrylamide gel was stained by ethidium bromide (upper panel) and Coomassie brilliant blue (lower panel). Lane 1, TmcA (80 pmol); lane 2, tRNAMet; lane 3, TmcA (40 pmol) and tRNAMet; lane 4, TmcA (80 pmol) and tRNAMet; lane 5, TmcA (160 pmol) and tRNAMet; lane 6, TmcA (80 pmol) and tRNAIle2; lane 7, tRNAIle2. Amount of tRNA was 80 pmol in all conditions.