Abstract
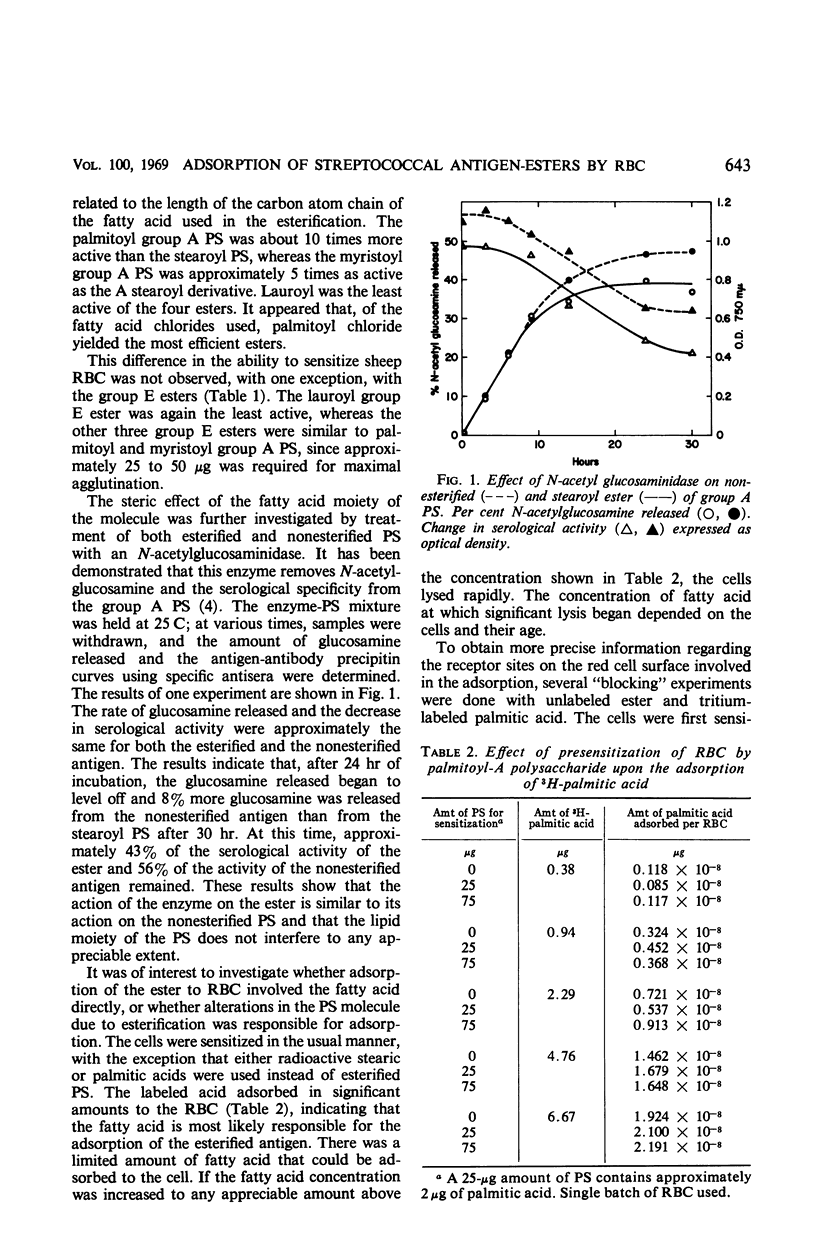
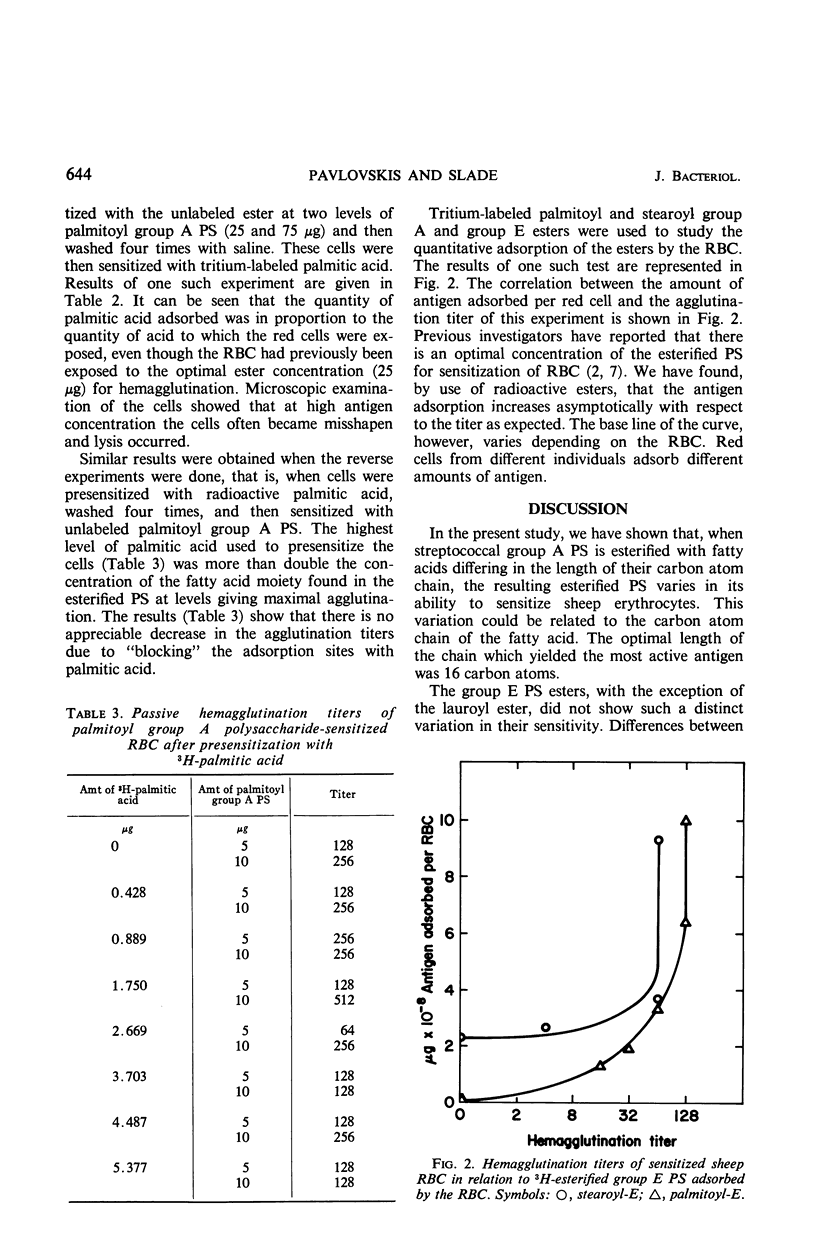
The streptococcal group A and E cell wall polysaccharide (PS) antigens were esterified under identical conditions with four fatty acid chlorides (lauroyl, myristoyl, palmitoyl, and stearoyl), varying from 12 to 18 carbon atoms. With group A PS, it was shown that the four resulting esters varied in their ability to sensitize red blood cells (RBC) to agglutination in the presence of specific antiserum. The most active was palmitoyl (16C) followed by myristoyl (14C). The least active was the lauroyl ester (12C). One-tenth as much palmitoyl ester was required as stearoyl group A PS ester. Such variation in the ability to sensitize RBC was not demonstrated with the group E esters, with the exception of the lauroyl ester which was the least active. Removal of N-acetylglucosamine from the esterified and the nonesterified group A PS by enzyme action resulted in a significant loss of serological activity of both antigens. No appreciable difference in the rate or total loss of activity was found in either case. It was demonstrated that both tritium-labeled stearic and palmitic acids and their respective PS esters were adsorbed in significant amounts to RBC. The results indicate that the esterified antigens were adsorbed to the RBC because of the presence of the fatty acid in the PS ester. Attempts to block the receptor sites on the red cell by presensitizing the cells with fatty acid were negative. Likewise, the adsorbed ester did not prevent the uptake of fatty acid at the levels tested. Tritium-labeled esterified group A PS and group E PS were used to show that the amount of antigen required to produce maximal agglutination was the same when cells from the same individual were used, whereas this was not the case when cells from different individuals were used. The amount of antigen required to produce maximal agglutination varied from one batch of sheep RBC to another. Once the optimal concentration of antigen was reached, any additional adsorption did not increase the titer of agglutination.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIES D. A., CRUMPTON M. J., MACPHERSON I. A., HUTCHISON A. M. The adsorption of bacterial polysaccharides by erythrocytes. Immunology. 1958 Apr;1(2):157–171. [PMC free article] [PubMed] [Google Scholar]
- HESS E. L., SLADE H. D. An electrophoretic examination of cell-free extracts from various serological types of group A hemolytic streptococci. Biochim Biophys Acta. 1955 Mar;16(3):346–353. doi: 10.1016/0006-3002(55)90237-7. [DOI] [PubMed] [Google Scholar]
- Hämmerling U., Westphal O. Synthesis and use of O-stearoyl polysaccharides in passive hemagglutination and hemolysis. Eur J Biochem. 1967 Mar;1(1):46–50. doi: 10.1007/978-3-662-25813-2_9. [DOI] [PubMed] [Google Scholar]
- MCCARTY M. Variation in the group-specific carbohydrate of group A streptococci. II. Studies on the chemical basis for serological specificity of the carbohydrates. J Exp Med. 1956 Nov 1;104(5):629–643. doi: 10.1084/jem.104.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NETER E. Bacterial hemagglutination and hemolysis. Bacteriol Rev. 1956 Sep;20(3):166–188. doi: 10.1128/br.20.3.166-188.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLADE H. D., SLAMP W. C. Cell-wall composition and the grouping antigens of Streptococci. J Bacteriol. 1962 Aug;84:345–351. doi: 10.1128/jb.84.2.345-351.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade H. D. Extraction of Cell-Wall Polysaccharide Antigen from Streptococci. J Bacteriol. 1965 Sep;90(3):667–672. doi: 10.1128/jb.90.3.667-672.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade H. D., Hammerling U. [Detection by hemagglutination of antibodies to group A and group E streptococci by the use of O-stearoyl derivatives of their cell wall carbohydrate-grouping antigens]. J Bacteriol. 1968 May;95(5):1572–1579. doi: 10.1128/jb.95.5.1572-1579.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer G. F., Wang E. T., Nichols J. H., Shear J. M. Relations between bacterial lipopolysaccharide structures and those of human cells. Ann N Y Acad Sci. 1966 Jun 30;133(2):566–579. doi: 10.1111/j.1749-6632.1966.tb52389.x. [DOI] [PubMed] [Google Scholar]


