Abstract
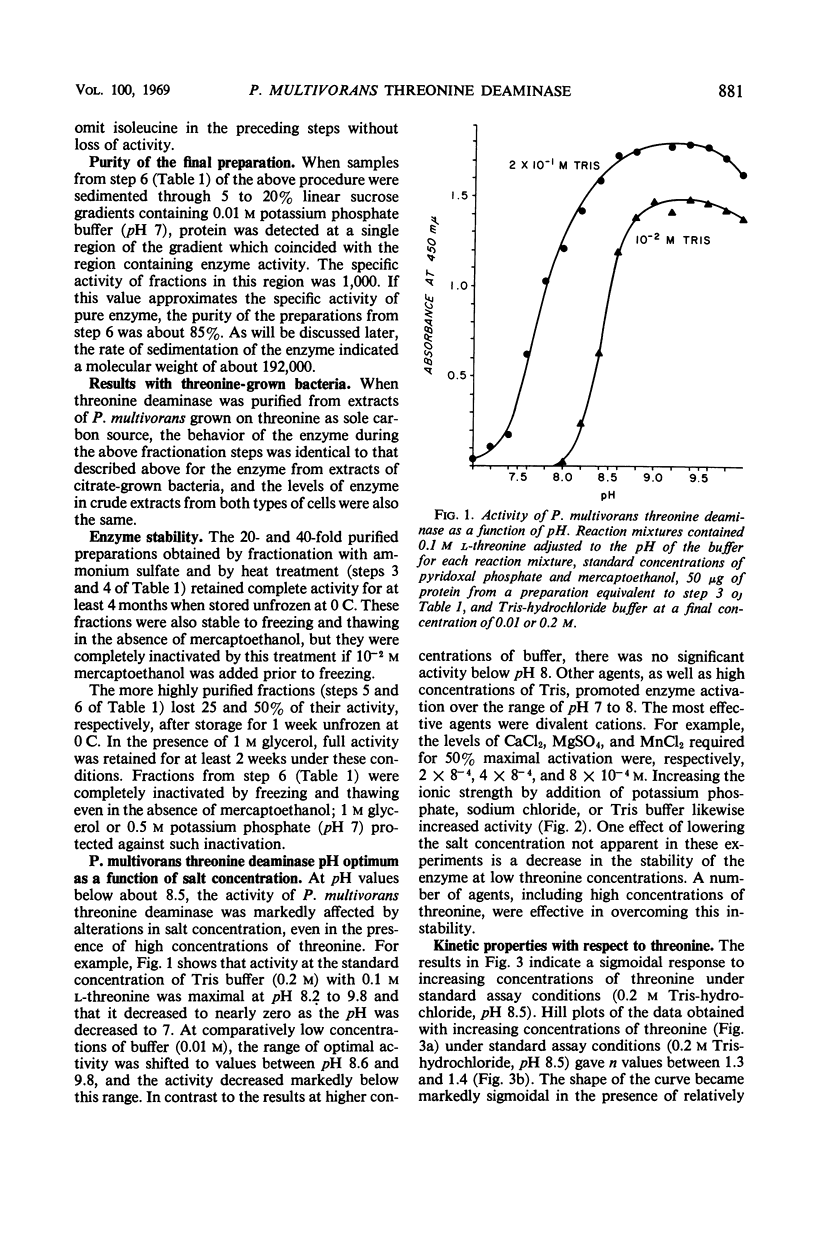
A threonine deaminase susceptible to inhibition by isoleucine was purified over 3,000-fold from extracts of Pseudomonas multivorans, a bacterium able to use threonine or α-ketobutyrate as sole source of carbon and energy. The enzyme was characterized with respect to molecular weight, dissociation to subunits, and apparent affinities for threonine, isoleucine, and several other ligands. Certain features of the enzyme including its reversible dissociation to subunits, its high constitutive activity, its marked stability, and high apparent orders of binding for threonine and isoleucine were unusual compared to those of isoleucine-inhibitable enzymes from other bacteria. These findings suggested some relationship between properties of the enzyme and the ability of P. multivorans to use threonine as sole carbon source. However, mutant studies ruled out a direct role of the enzyme in threonine catabolism and indicated that another enzyme, threonine dehydrogenase, is essential for growth on threonine.
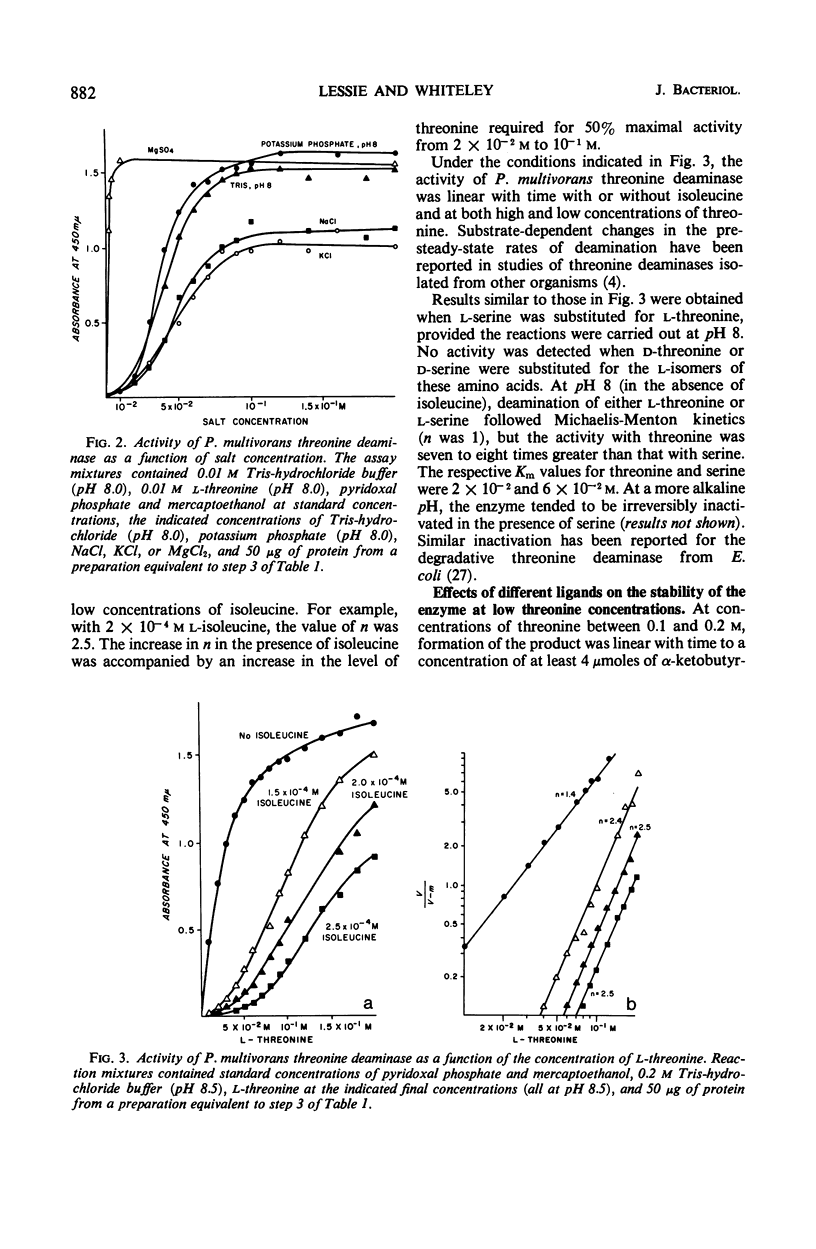
Full text
PDF


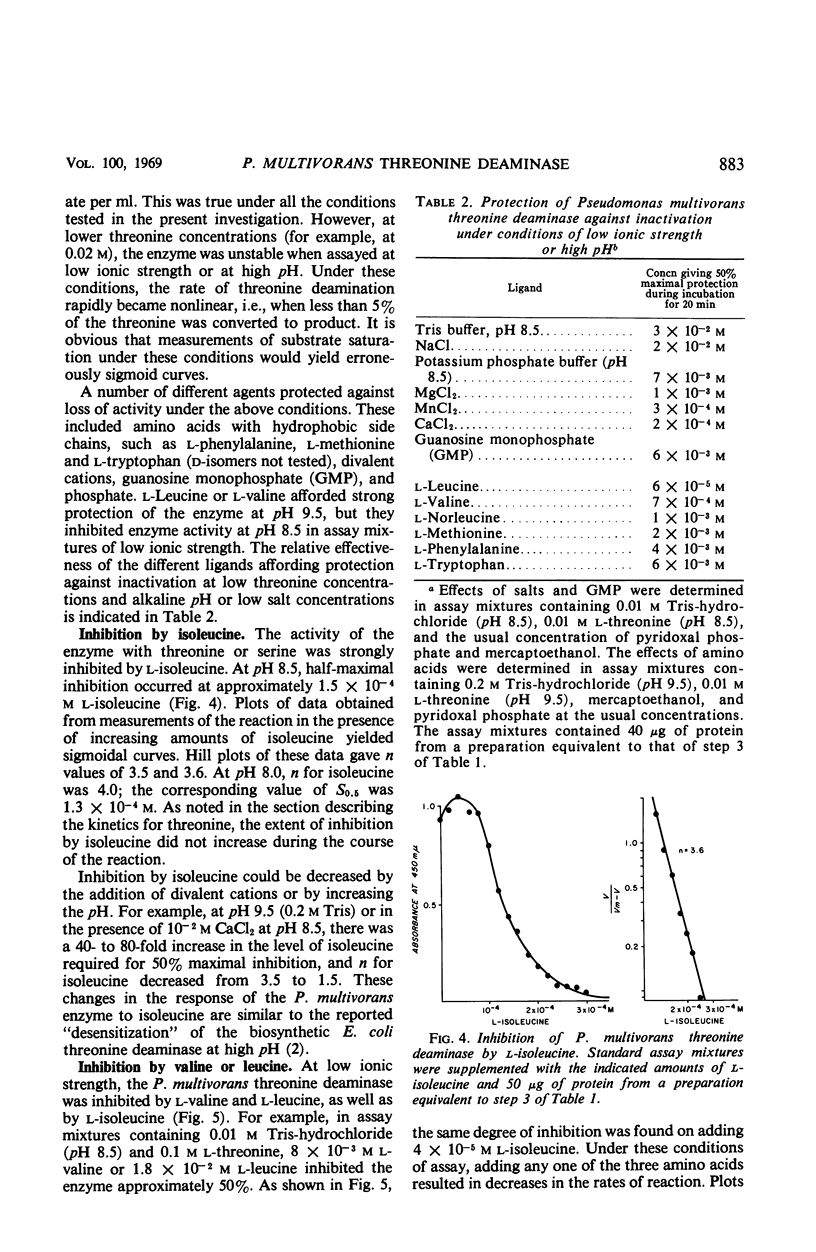




Selected References
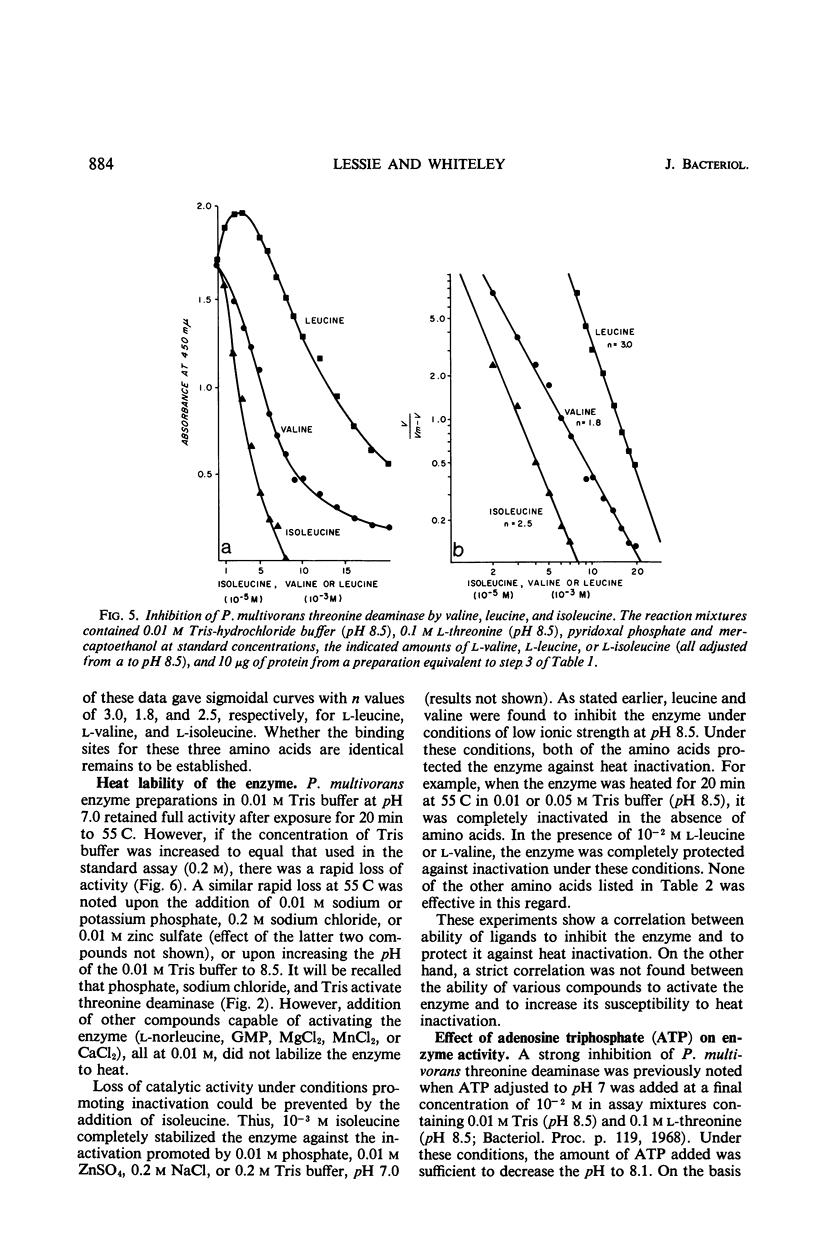
These references are in PubMed. This may not be the complete list of references from this article.
- Burns R. O., Zarlengo M. H. Threonine deaminase from Salmonella typhimurium. I. Purification and properties. J Biol Chem. 1968 Jan 10;243(1):178–185. [PubMed] [Google Scholar]
- CHANGEUX J. P. The feedback control mechanisms of biosynthetic L-threonine deaminase by L-isoleucine. Cold Spring Harb Symp Quant Biol. 1961;26:313–318. doi: 10.1101/sqb.1961.026.01.037. [DOI] [PubMed] [Google Scholar]
- Datta P. Purification and feedback control of threonine deaminase activity of Rhodopseudomonas spheroides. J Biol Chem. 1966 Dec 25;241(24):5836–5844. [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON K. D., LAVER W. G., NEUBERGER A. Initial stages in the biosynthesis of porphyrins. 2. The formation of delta-aminolaevulic acid from glycine and succinyl-coenzyme A by particles from chicken erythrocytes. Biochem J. 1958 Sep;70(1):71–81. doi: 10.1042/bj0700071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinathan K. P., DeMoss R. D. The role of tris(hydroxymethyl)aminomethane and cysteine in the dissociation of tryptophanase. Biochemistry. 1968 May;7(5):1685–1691. doi: 10.1021/bi00845a010. [DOI] [PubMed] [Google Scholar]
- HAYAISHI O., GEFTER M., WEISSBACH H. Adenosine diphosphate-dependent threonine dehydrase activity in extracts of Clostridium tetanomorphum. J Biol Chem. 1963 Jun;238:2040–2044. [PubMed] [Google Scholar]
- HIRATA M., TOKUSHIGE M., INAGAKI A., HAYAISHI O. NUCLEOTIDE ACTIVATION OF THREONINE DEAMINASE FROM ESCHERICHIA COLI. J Biol Chem. 1965 Apr;240:1711–1717. [PubMed] [Google Scholar]
- Hatfield G. W., Umbarger H. E. A time-dependent activation of threonine deaminase. Biochem Biophys Res Commun. 1968 Nov 8;33(3):397–401. doi: 10.1016/0006-291x(68)90584-6. [DOI] [PubMed] [Google Scholar]
- Higgins I. J., Turner J. M., Willetts A. J. Enzyme mechanism of aminoacetone metabolism by micro-organisms. Nature. 1967 Aug 19;215(5103):887–888. doi: 10.1038/215887a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lessie T. G., Neidhardt F. C. Formation and operation of the histidine-degrading pathway in Pseudomonas aeruginosa. J Bacteriol. 1967 Jun;93(6):1800–1810. doi: 10.1128/jb.93.6.1800-1810.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Maeba P., Sanwal B. D. The allosteric threonine deaminase of Salmonella. Kinetic model for the native enzyme. Biochemistry. 1966 Feb;5(2):525–536. doi: 10.1021/bi00866a019. [DOI] [PubMed] [Google Scholar]
- McGilvray D., Morris J. G. Utilization of L-threonine by a species of Arthrobacter. A novel catabolic role for "aminoacetone synthase". Biochem J. 1969 May;112(5):657–671. doi: 10.1042/bj1120657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa A., Hayaishi O. On the mechanism of activation of L-threonine deaminase from Clostridium tetanomorphum by adenosine diphosphate. J Biol Chem. 1967 Mar 25;242(6):1146–1154. [PubMed] [Google Scholar]
- Robichon-Szulmajster H., Magee P. T. The regulation of isoleucine-valine biosynthesis in Saccharomyces cerevisiae. I. Threonine deaminase. Eur J Biochem. 1968 Feb;3(4):492–501. doi: 10.1111/j.1432-1033.1967.tb19558.x. [DOI] [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Isoleucine and valine metabolism in Escherichia coli. VII. A negative feedback mechanism controlling isoleucine biosynthesis. J Biol Chem. 1958 Aug;233(2):415–420. [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Threonine deamination in Escherichia coli. II. Evidence for two L-threonine deaminases. J Bacteriol. 1957 Jan;73(1):105–112. doi: 10.1128/jb.73.1.105-112.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanquickenborne A., Phillips A. T. Purification and regulatory properties of the adenosine diphosphate-activated threonine dehydratase. J Biol Chem. 1968 Mar 25;243(6):1312–1319. [PubMed] [Google Scholar]
- WOOD W. A., GUNSALUS I. C. Serine and threonine desaminaes of Escherichia coli; activators for a cell-free enzyme. J Biol Chem. 1949 Nov;181(1):171–182. [PubMed] [Google Scholar]
- Whanger P. D., Phillips A. T., Rabinowitz K. W., Piperno J. R., Shada J. D., Wood W. A. The mechanism of action of 5'-adenylic acid-activated threonine dehydrase. II. Protomer-oligomer interconversions and related properties. J Biol Chem. 1968 Jan 10;243(1):167–173. [PubMed] [Google Scholar]
- Whiteley H. R., Tahara M. Threonine deaminase of Clostridium tetanomorphum. I. Purification and properties. J Biol Chem. 1966 Nov 10;241(21):4881–4889. [PubMed] [Google Scholar]
- Whiteley H. R. Threonine deaminase of Clostridium tetanomorphum. II. Dissociation to subunits. J Biol Chem. 1966 Nov 10;241(21):4890–4898. [PubMed] [Google Scholar]
- Zarlengo M. H., Robinson G. W., Burns R. O. Threonine deaminase from Salmonella typhimurium. II. The subunit structure. J Biol Chem. 1968 Jan 10;243(1):186–191. [PubMed] [Google Scholar]


