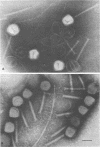
Abstract
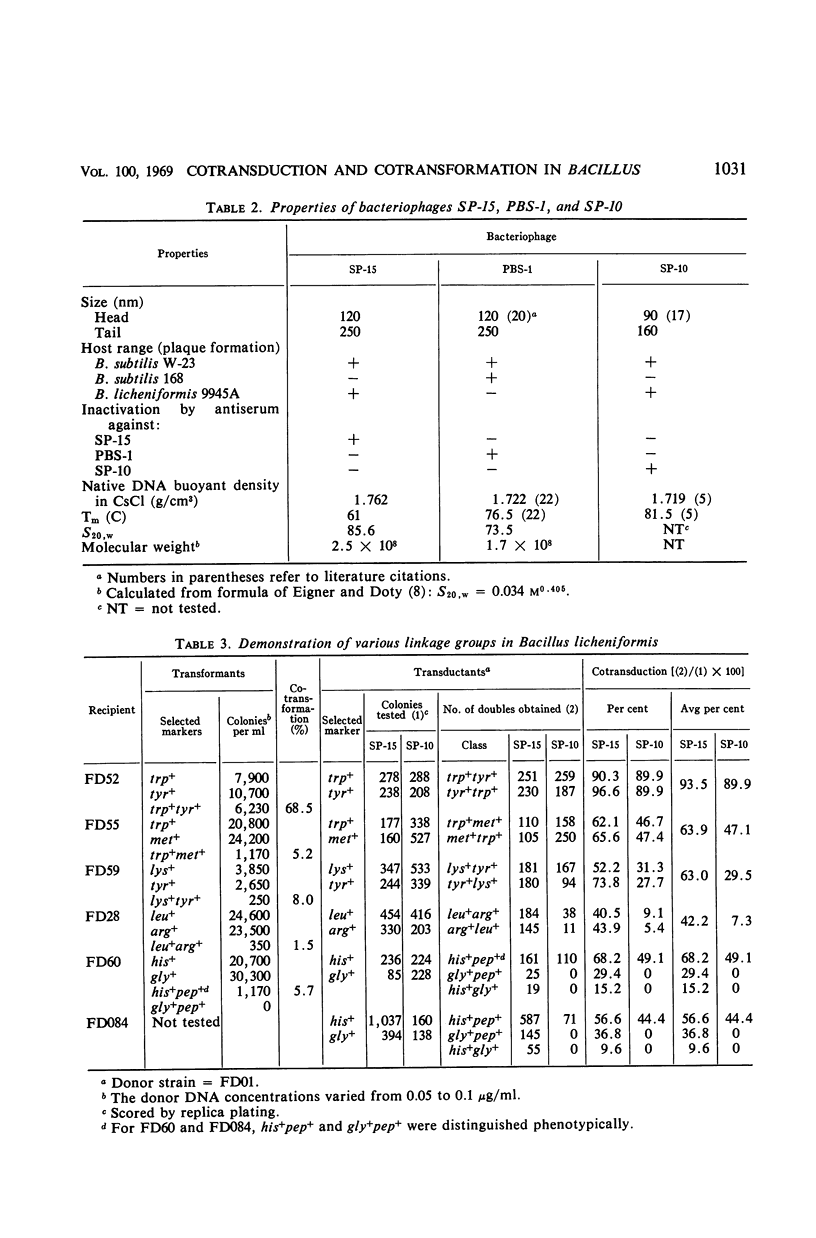
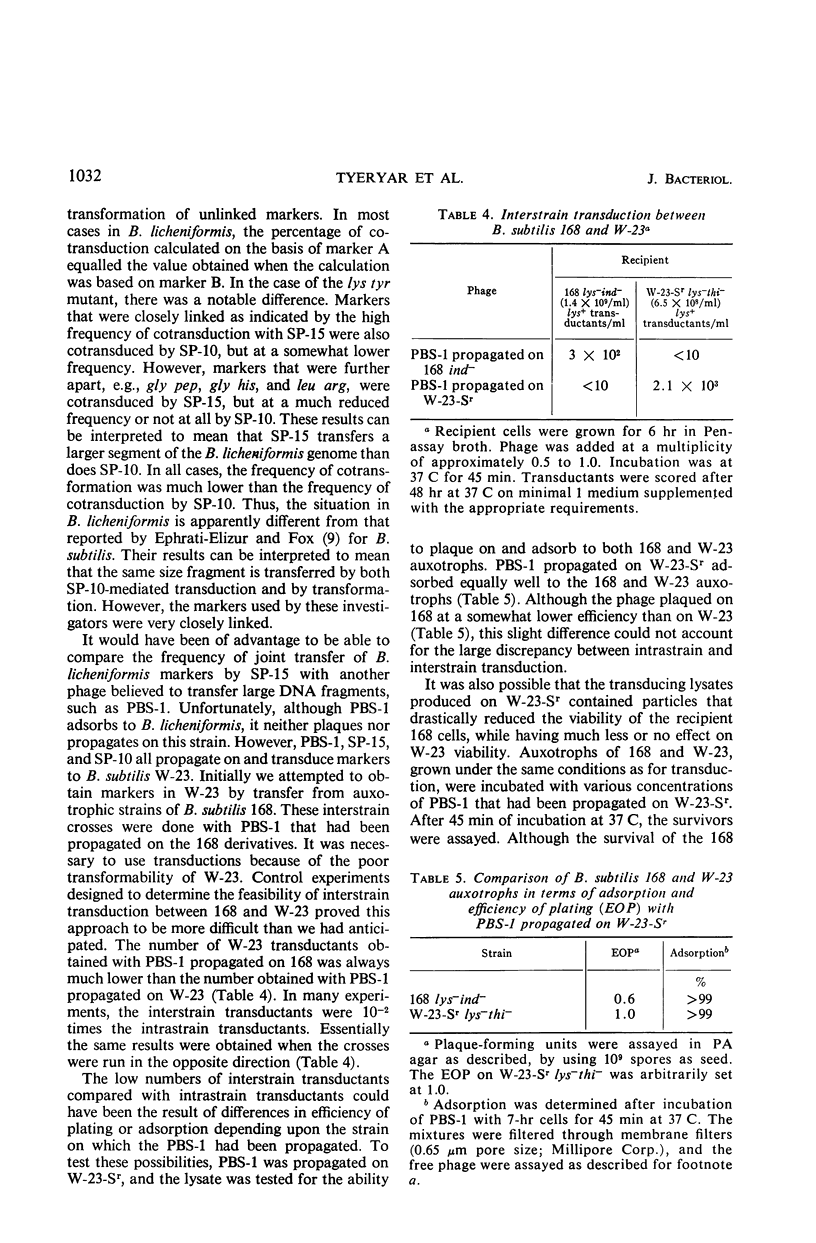
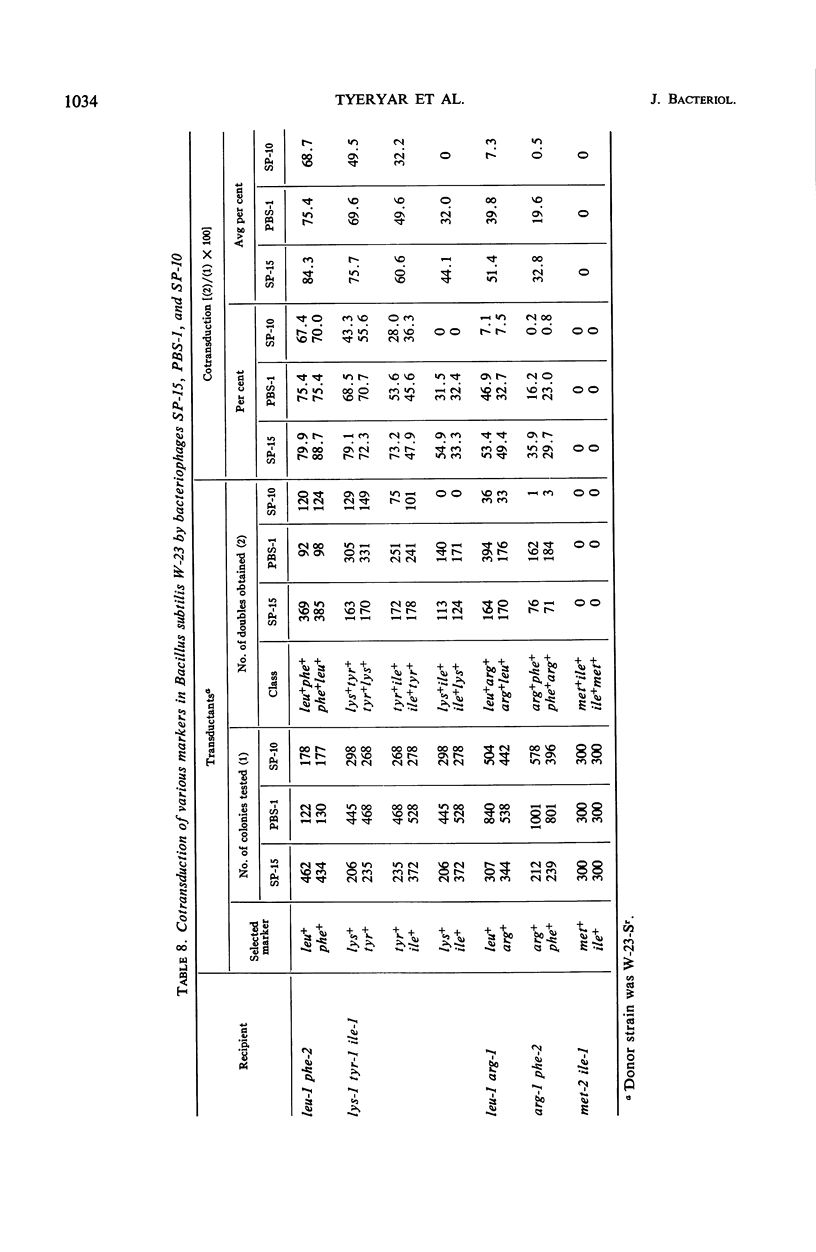
Bacteriophage SP-15, a large generalized transducing phage of Bacillus, was compared with phages PBS-1 and SP-10 for the ability to cotransduce pairs of genetic markers exhibiting different degrees of linkage. When auxotrophs of B. subtilis W-23 were used as recipients, SP-15 and PBS-1 effected a much higher frequency of cotransduction than did SP-10 with markers that were not closely linked. With more closely linked loci, the differences were not as great. SP-15 cotransduced linked markers at a higher mean frequency than PBS-1, suggesting that SP-15 is able to transfer a larger fragment of the Bacillus genome than any phage heretofore described. The frequency of the joint transfer of genetic markers in B. licheniformis was lower via transforming deoxyribonucleic acid than by transduction with phage SP-10. The availability of three procedures for genetic exchange—transduction by SP-15 and SP-10 as well as transformation—each of which reveals a different degree of linkage, makes B. licheniformis 9945A especially amenable to genetic analysis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANAGNOSTOPOULOS C., CRAWFORD I. P. Transformation studies on the linkage of markers in the tryptophan pathway in Bacillus subtilis. Proc Natl Acad Sci U S A. 1961 Mar 15;47:378–390. doi: 10.1073/pnas.47.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenbern R. A. Apparent genomic mapping of Staphylococcus aureus by a new method. Biochem Biophys Res Commun. 1966 Nov 11;25(3):346–353. doi: 10.1016/0006-291x(66)90784-4. [DOI] [PubMed] [Google Scholar]
- BARAT M., ANAGNOSTOPOULOS C., SCHNEIDER A. M. LINKAGE RELATIONSHIPS OF GENES CONTROLLING ISOLEUCINE, VALINE, AND LEUCINE BIOSYNTHESIS IN BACILLUS SUBTILIS. J Bacteriol. 1965 Aug;90:357–369. doi: 10.1128/jb.90.2.357-369.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOTT K., STRAUSS B. THE CARRIER STATE OF BACILLUS SUBTILIS INFECTED WITH THE TRANSDUCING BACTERIOPHAGE SP10. Virology. 1965 Feb;25:212–225. doi: 10.1016/0042-6822(65)90200-x. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Goldthwaite C., Smith I., Marmur J. Genetic mapping in Bacillus subtilis. J Mol Biol. 1967 Jul 14;27(1):163–185. doi: 10.1016/0022-2836(67)90358-0. [DOI] [PubMed] [Google Scholar]
- Eigner J., Doty P. The native, denatured and renatured states of deoxyribonucleic acid. J Mol Biol. 1965 Jul;12(3):549–580. doi: 10.1016/s0022-2836(65)80312-6. [DOI] [PubMed] [Google Scholar]
- Goldberg I. D., Bryan T. Productive infection of Bacillus subtilis 168, with bacteriophage SP-10, dependent upon inducing treatments. J Virol. 1968 Aug;2(8):805–812. doi: 10.21236/ad0686354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer V. CONCENTRATION AND ISOLATION OF AUXOTROPHIC MUTANTS OF SPOREFORMING BACTERIA. J Bacteriol. 1960 Feb;79(2):309–310. doi: 10.1128/jb.79.2.309-310.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY M. S., PRITCHARD R. H. UNSTABLE LINKAGE BETWEEN GENETIC MARKERS IN TRANSFORMATION. J Bacteriol. 1965 May;89:1314–1321. doi: 10.1128/jb.89.5.1314-1321.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. S. Physical and mapping properties of distant linkages between genetic markers in transformation of Bacillus subtilis. Mol Gen Genet. 1967;99(4):333–349. doi: 10.1007/BF00330909. [DOI] [PubMed] [Google Scholar]
- Kelly M. S. The causes of instability of linkage in transformation of Bacillus subtilis. Mol Gen Genet. 1967;99(4):350–361. doi: 10.1007/BF00330910. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- OKUBO S., STODOLSKY M., BOTT K., STRAUSS B. SEPARATION OF THE TRANSFORMING AND VIRAL DEOXYRIBONUCLEIC ACIDS OF A TRANSDUCING BACTERIOPHAGE OF BACILLUS SUBTILIS. Proc Natl Acad Sci U S A. 1963 Oct;50:679–686. doi: 10.1073/pnas.50.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMIG W. R. Infection of Bacillus subtilis with phenol-extracted bacteriophages. Virology. 1962 Apr;16:452–459. doi: 10.1016/0042-6822(62)90226-x. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI I., MARMUR J. Replacement of thymidylic acid by deoxyuridylic acid in the deoxyribonucleic acid of a transducing phage for Bacillus subtilis. Nature. 1963 Feb 23;197:794–795. doi: 10.1038/197794a0. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI I. Transducing phages for Bacillus subtilis. J Gen Microbiol. 1963 May;31:211–217. doi: 10.1099/00221287-31-2-211. [DOI] [PubMed] [Google Scholar]
- TAYLOR M. J., THORNE C. B. TRANSDUCTION OF BACILLUS LICHENIFORMIS AND BACILLUS SUBTILIS BY EACH OF TWO PHAGES. J Bacteriol. 1963 Sep;86:452–461. doi: 10.1128/jb.86.3.452-461.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNE C. B. Transduction in Bacillus subtilis. J Bacteriol. 1962 Jan;83:106–111. doi: 10.1128/jb.83.1.106-111.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I. Joint transfer of genetic markers in Bacillus subtilis. J Bacteriol. 1966 Jan;91(1):101–105. doi: 10.1128/jb.91.1.101-105.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. J., Thorne C. B. Concurrent changes in transducing efficiency and content of transforming deoxyribonucleic acid in Bacillus subtilis bacteriophage SP-10. J Bacteriol. 1966 Jan;91(1):81–88. doi: 10.1128/jb.91.1.81-88.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne C. B., Stull H. B. Factors affecting transformation of Bacillus licheniformis. J Bacteriol. 1966 Mar;91(3):1012–1020. doi: 10.1128/jb.91.3.1012-1020.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyeryar F. J., Jr, Lawton W. D., MacQuillan A. M. Sequential replication of the chromosome of Bacillus licheniformis. J Bacteriol. 1968 Jun;95(6):2062–2069. doi: 10.1128/jb.95.6.2062-2069.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]