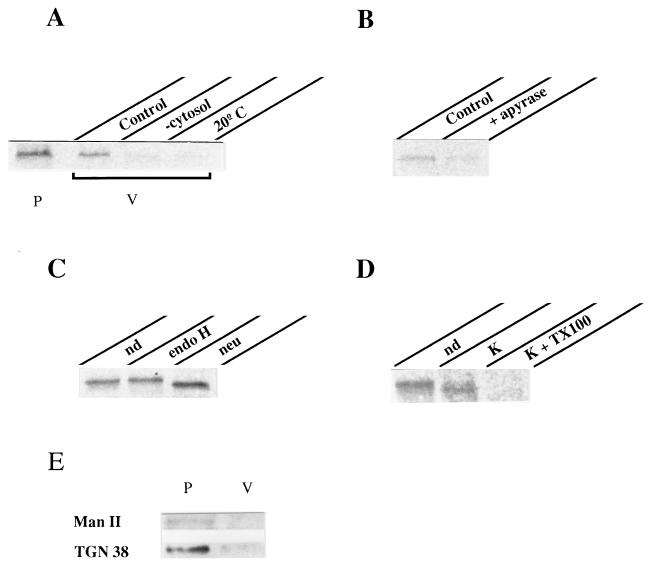
Figure 4.
Release of post-Golgi vesicles from permeabilized cells. The standard assay consisted of permeabilized cells containing radiolabeled VSV-G accumulated in the TGN, exogenous cytosol, GTP, and ATP-generating system (see Materials and Methods). Incubation took place at 37°C for 2 h. The vesicle fraction (V) was separated from the permeabilized cells (P) by centrifugation and its content in VSV-G determined by immunoprecipitation and SDS/PAGE analysis. (A) Comparison of the amount of VSV-G released into vesicles (V) vs. that remaining within the cells (P) in control conditions. VSV-G released in incubations lacking exogenous cytosol (−cytosol) or performed at low temperature (20°C) is shown. (B) ATP requirement: incubation was carried out in the presence of 1 mM GTPγS instead of GTP and additionally with or without (control) 0.6 units/ml apyrase. (C) Glycosylation of released VSV-G: VSV-G immunoprecipitated from vesicles was subjected or not (nondigested, nd) to either endoglycosidase H (endo H) or neuraminidase (neu) digestion before SDS/PAGE. (D) Vesicle integrity: released vesicles were incubated or not (nd) on ice for 30 min with 0.5 mg/ml proteinase K (K) in the presence or absence of 1% (vol/vol) Triton X-100 (TX100). VSV-G was then immunoprecipitated with 8G5F11 antibody against its extracytoplasmic domain. (E) Detection of both α-1,2-mannosidase II (Man II) and TGN38: 30 μg of membrane proteins from both fractions (P, V) were resolved by SDS/PAGE and determined by Western blot analysis.