Abstract
The initiation of DNA replication in Saccharomyces cerevisiae requires the protein product of the CDC45 gene. We report that although Cdc45p is present at essentially constant levels throughout the cell cycle, it completes its initiation function in late G1, after START and prior to DNA synthesis. Shortly after mitosis, cells prepare for initiation by assembling prereplicative complexes at their replication origins. These complexes are then triggered at the onset of S phase to commence DNA replication. Cells defective for CDC45 are incapable of activating the complexes to initiate DNA replication. In addition, Cdc45p and Cdc7p/Dbf4p, a kinase implicated in the G1/S phase transition, are dependent on one another for function. These data indicate that CDC45 functions in late G1 phase in concert with CDC7/DBF4 to trigger initiation at replication origins after the assembly of the prereplicative complexes.
Maintenance of genome integrity is essential for cell viability. Two events in the cell cycle critical to this task are the replication of chromosomes during S phase and their segregation in mitosis. During S phase, the cell must assure not only that replication is completed before mitosis, but also that each origin initiates replication no more than once per cell cycle. Saccharomyces cerevisiae has proven to be an exceptionally tractable system in which to dissect the mechanism and regulation of replication initiation. Powerful genetic techniques in addition to well-defined origins of replication have facilitated the identification of many yeast components required for the initiation of DNA replication. These include the origin recognition complex (ORC) that binds to yeast origins (1–6), a family of structurally and functionally similar proteins called MCM (minichromosome maintenance) proteins (7–10), Cdc6p (11), and two kinases, Cdc7p (12–14) and Cdc28p (15).
Genomic footprint analysis of the proteins assembled at yeast origins during the cell cycle has suggested a two-step model for the initiation of DNA replication (16). Many of the initiation proteins mentioned above have been implicated in one or both of these steps. ORC is believed to be bound to origins throughout the cell cycle. In the first step, which occurs shortly after mitosis, additional proteins are thought to join ORC to form prereplicative complexes (pre-RCs) at origins in preparation for S phase (16). One of these proteins might be Cdc6p because it is required for both assembly and maintenance of the pre-RC (17, 18). Other candidates include the six members of the MCM family of proteins (MCM2, MCM3, MCM5/CDC46, CDC47, CDC54, and MCM6) (19–21). In yeast, several of these proteins have been shown to shuttle between the cytoplasm and nucleus, entering the nucleus coincident with pre-RC formation (7, 8, 16, 22). In Xenopus replication extracts, MCM homologs appear to be loaded onto chromatin in an XCdc6p-dependent manner in preparation for S phase (23). Formation of the pre-RC, although necessary, is not sufficient to initiate replication in yeast. Cells must also pass through the G1 commitment point START before they can execute a second step that presumably activates the pre-RC and triggers the initiation of DNA replication (reviewed in refs. 24–26). This step is thought to require two kinases, Cdc7p (12) and Cdc28p (15), in association with their respective regulatory subunits, Dbf4p (12) and Clb5p/Clb6p (27, 28). The entry into S phase correlates with replacement of the pre-RC by an ORC-like postreplicative complex (post-RC) (16). This transition presumably reflects the simultaneous activation and disassembly of the pre-RC during initiation and we refer to this transition as the triggering of the pre-RC.
CDC45 is an essential gene required for DNA replication (29–31). Cdc45p has been localized to the nucleus (29) and CDC45 mRNA expression is periodic, peaking during the G1/S transition (32). Three different experiments have suggested it functions in the initiation of replication. First, mutations in CDC45 have been shown to interact genetically with mutations in several initiation components, including members of the MCM family and components of ORC (31–33). Second, mutant cdc45 cells lose plasmids at an elevated frequency in a manner that can be suppressed by the addition of multiple origins to the plasmids (29, 31, 32). Finally, analysis of replication intermediates in cdc45-1 cells shows a reduced frequency of firing at individual origins of replication (31).
We have studied CDC45 further to understand its role in replication initiation. We show that despite the periodicity of its mRNA expression, Cdc45p is present at relatively constant levels throughout the cell cycle, and that it completes its initiation function in late G1, after START and prior to elongation of DNA replication. Cells defective for CDC45 function are unable to trigger pre-RCs once they are formed. In addition, Cdc45p and Cdc7p kinases are dependent on each other for execution of their replication functions. A similar interdependent relationship was observed between Cdc45p and Dbf4p, the regulatory subunit for Cdc7p kinase. These data lead to the conclusion that CDC45 functions in conjunction with Cdc7p/Dbf4p to trigger pre-RCs during the initiation of DNA replication.
MATERIALS AND METHODS
Media and Budding Index.
Yeast extract/peptone (YEP) medium (34) was supplemented with 2% dextrose (YEPD). Unless otherwise stated, cells were grown at 30°C. To arrest cells, α-factor was used at 50 ng/ml (for bar1 cells) or 10 μg/ml (for BAR1 cells); hydroxyurea (HU) was used at 0.2 M. For analysis of budding index, cells with bud diameter less than 50% that of the mother cell were scored as small-budded cells, whereas those with a bud diameter greater than 50% that of the mother cell were scored as large-budded cells. In our strains the appearance of small buds coincides with the onset of replication (18).
Plasmids and Strains.
The cdc45-1 yeast strain used to order CDC45 function relative to START and replication elongation is YJL556 (DBY2027, MATa cdc45-1 ade2-1 lys2-801 leu2-3, 112 ura3-52). The related wild type strains used for controls were YJL1086 (DBY1705, MATα leu2-3, 112 ura3-52 lys2-801) and YJL1085 (DBY640, MATa ade2-1 gal- mal-). The strain used to functionally order CDC45 and CDC7 is YJL1907 (MATa cdc45-1 cdc7-4 leu2-3, 112 ura3-52 ade2-1 lys2-801 trp1-289). The strain used to functionally order CDC45 and DBF4 is YJL1908 (MATa cdc45-1, dbf4-1, leu2-3, 112, trp1-289, ura3-52, bar::LEU2). The strains used for genomic footprinting are YJL1496 (MATa cdc45-1 ade2-1 lys2-801 leu2-3, 112 ura3-52 bar1::LEU2) and YJL310 (MATa leu2-3, 112 ura3-52 trp1-289 bar1::LEU2). Epitope-tagged CDC45 was produced by first cloning a blunt-ended BsaI–SphI CDC45 genomic fragment into the XhoI and NotI sites of pRS306. The sequence 5′-GGCGGCCGCGCACCGGTG-3′ containing the NotI and SgrAI restriction sites was inserted by oligo-directed mutagenesis (35) immediately 5′ of the CDC45 stop codon and the mutagenesis confirmed by sequencing. Into the NotI site, a NotI fragment encoding three tandem copies of the hemagglutinin epitope (HA)3 (36) was inserted in-frame with the CDC45 ORF, yielding the plasmid pJO05. The tagged CDC45 gene was substituted for the wild-type copy by two-step gene replacement (34) yielding yeast strain YJL1906 (MATa CDC45-HA3 ura3-52 trp1-289 leu2-3, 112 bar1::LEU2). The desired replacement was confirmed by Southern blot analysis (35).
Yeast Protein Preparations for Western Blot Analysis.
Cells (4 ml) at OD600 0.5–1.0 were pelleted and lysed by vortex mixing and boiling with 200 μl 0.5-mm glass beads (Biospec Products, Bartlesville, OK) and 150 μl SDS/PAGE loading buffer (5% glycerol/0.5% SDS/64 mM Tris⋅Cl/120 mM DTT) with protease inhibitors (4 mM EDTA/1 mM phenylmethylsulfonyl fluoride/1 μg/ml leupeptin/10 μg/ml aprotinin/2 mM benzamidine/1 μg/ml pepstatin A). The soluble protein was quantified by Bradford assay (Bio-Rad) by using BSA fraction V (Sigma) as a standard. Forty micrograms of each sample were separated on SDS/PAGE and transferred to a Micron Separations (Westboro, MA) Nitrobind 0.22-μm membrane. The membrane was probed with 12CA5 anti-HA mAb diluted at 1:2,000 [Babco ascites (Babco, Richmond, CA)] or anti-Sec61 antibodies diluted at 1:6,000 (antiserum a gift of Sylvia Sanders, Massachusetts Institute of Technology and used as a loading control), followed by horseradish peroxidase-conjugated goat anti-mouse or donkey anti-rabbit secondary antibodies (Bio-Rad), respectively. Immunoblots were developed with the Amersham ECL system.
Functional Ordering and Flow Cytometry.
Cells were released from arrests by filtering, washing with at least two volumes of medium and resuspending in fresh medium prewarmed or precooled to the appropriate temperature and containing the appropriate drug. cdc45-1 arrests were obtained by incubation at 11°C for 16 h; cdc7-4 and dbf4-1 arrests were achieved by incubation at 38°C for 2.5–3 h. Arrests at START and prior to replication elongation were obtained by incubation of cells in the presence of α-factor and HU, respectively, for 2 h at 30°C. Samples were taken for flow cytometry as described (18).
Genomic Footprinting.
Genomic footprinting of the 2-micron origin of replication was performed essentially as described (37, 38). The exposure time used for the cdc45-1 genomic footprints was five times that of the CDC45 footprints. This adjustment was necessary because cdc45-1 cells, which are defective in maintaining plasmids (29, 31, 32), presumably contain, on average, a lower level of 2-micron plasmids than CDC45 cells.
RESULTS
Cdc45p Is Maintained at Constant Levels in Cycling Cells.
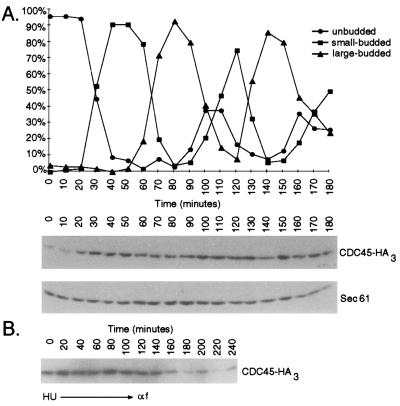
Previous studies have shown that CDC45 mRNA expression is periodic, with maximal levels appearing at the G1/S phase boundary (32). To determine whether Cdc45p levels fluctuated in parallel with mRNA levels, we constructed a strain in which three tandem copies of the HA epitope (36) were fused to the C terminus of the endogenous protein (Cdc45p-HA3). The tagged protein appears to function normally because this strain grew equivalently to a wild-type strain and progressed normally through the cell cycle as indicated by its budding index and flow cytometry profile (data not shown). CDC45-HA3 cells were synchronously released from an α-factor-induced G1 arrest, and the amount of the tagged protein was assessed by immunoblotting with anti-HA mAbs. Good synchrony was maintained for two cell cycles as monitored by budding index (Fig. 1A) and flow cytometry (data not shown). With the exception of the earliest time points, the level of Cdc45p-HA3 was relatively constant during both cell cycles (varying less than 2.5-fold after normalization for differences in lane loading). The elevated protein levels present in G1 phase of the second cell cycle (100 and 110 min) suggested that the low amounts observed at the beginning of the time course (0 and 10 min) did not represent normal G1 phase levels but arose from the prolonged α-factor arrest used to synchronize the cells.
Figure 1.
Cdc45p is present at constant levels in cycling cells. (A) CDC45-HA3 cells were synchronously released from an α-factor arrest. Whole-cell extracts were made at various time points and total cell protein extracts were made, resolved by SDS/PAGE, and immunoblotted with antibodies against the HA epitope (Lower). Blots were reprobed with anti-Sec61p antibodies as a loading control (18). Cell synchrony was evaluated by determining percent of unbudded, small-budded, and large-budded cells. (B) CDC45-HA3 cells were synchronously released from a HU arrest into medium containing α-factor. Total cell protein extracts were analyzed as described above. Cells reached the α-factor block 120 min after release from HU as monitored by flow cytometry (data not shown).
To confirm this notion, cells were synchronously released from an S phase arrest induced by HU and allowed to progress into an α-factor block in the next cell cycle (Fig. 1B). Within 120 min of release from the HU block, cells were fully arrested in G1 as monitored by flow cytometry (data not shown), but still maintained the elevated levels of Cdc45p seen in cycling cells. Eventually, after more prolonged arrest, protein levels dropped to the low levels seen at 0 min in Fig. 1A. Thus, we conclude that Cdc45p levels remain constant in cycling cells. Because the protein levels are maintained despite periodic fluctuations in CDC45 mRNA levels(32), we also infer that Cdc45p is relatively stable in these cells.
Cdc45p Completes its Replication Function Before DNA Elongation Occurs.
To examine when Cdc45p is needed for DNA replication, we determined the order in which CDC45 functions relative to two cell cycle events: the elongation phase of DNA replication in S phase and the passage through START in G1 phase. Experiments with similar aims have been described (31) but were complicated by incomplete inactivation of CDC45 function and inability to recover cycling cells from the cdc45-1 arrest (see Discussion).
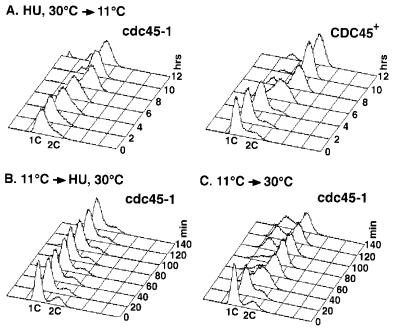
In our first set of experiments we asked whether CDC45 function is necessary for replication once cells have entered S phase. We released cdc45-1 and CDC45 cultures arrested in S phase by HU, which blocks replication elongation, into the cdc45-1-restrictive temperature of 11°C and monitored DNA content by flow cytometry. As seen in Fig. 2A, both cdc45-1 and CDC45 cells replicated after release from the HU block. This finding suggests that Cdc45p is not required for DNA elongation and executes its function independently of this step.
Figure 2.
DNA elongation is dependent on CDC45 function. (A) cdc45-1 and CDC45 cultures were arrested in S phase with HU-containing media at 30°C. At 0 h, the cultures were released from the S phase arrest and shifted to 11°C. Samples were monitored flow cytometry and budding index. (B) A cdc45-1 culture was arrested by incubation in medium at 11°C, the cdc45-1-restrictive temperature. At 0 min, the culture was shifted to 30°C and HU was added. Samples were monitored by flow cytometry and budding index. (C) Samples were taken as in B, except no HU was added.
To determine whether replication elongation depends on execution of Cdc45p function, we performed the reciprocal experiment. cdc45-1 cells were arrested at 11°C and released into HU-containing medium at 30°C. Flow cytometry showed that cdc45-1 cells were unable to replicate after release from the 11°C block, retaining a 1C DNA content (Fig. 2B). In contrast, cdc45-1 cells released at 30°C in the absence of HU replicated within 40 min, confirming that the cdc45-1 arrest was reversible (Fig. 2C). Thus, we conclude that DNA elongation is dependent on prior execution of a CDC45 function, consistent with Cdc45p playing a role in the initiation of DNA replication (29, 31, 32).
It should be noted, however, that the cdc45-1 cells released from HU into 11°C replicated slightly slower than the wild-type strain and that many of these cells did not proceed through mitosis (data not shown). These observations might be explained by impaired initiation at late replication origins resulting in incomplete replication and checkpoint arrest. However, we cannot rule out a role for CDC45 in the efficient and complete progression of replication forks during elongation.
Cdc45p Is Required After START for DNA Replication.
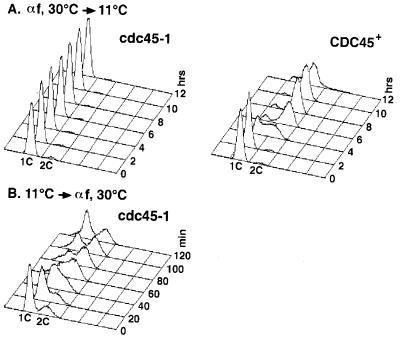
Previous experiments have suggested that CDC45 function is needed after START for optimal DNA replication (31). To determine whether CDC45 function is actually essential for replication after START, we released cdc45-1 and CDC45 cells from a START arrest imposed by α factor at 30°C into medium at the cdc45-1 restrictive temperature of 11°C. As shown in Fig. 3A, cdc45-1 cells maintained a 1C DNA content 12 h after release from α factor, whereas the CDC45 wild-type strain replicated and acquired a 2C DNA content within 6 h following release. The cdc45-1 cells did release from the α-factor arrest, because >90% of the cells were observed as large-budded cells (data not shown). This result indicates that Cdc45p function is required after START for replication and that execution of this function is dependent on passage through START.
Figure 3.
CDC45 function is dependent on START and is essential for DNA replication. (A) cdc45-1 and CDC45 cultures were arrested in G1 phase with α-factor-containing medium at 30°C. At 0 h, the cultures were released from the START arrest and shifted to 11°C. Samples were monitored by flow cytometry and budding index. (B) A cdc45-1 culture was arrested by incubation in medium at 11°C, the cdc45-1-restrictive temperature. At 0 min, the culture was shifted to 30°C and α factor was added. Samples were monitored by flow cytometry and budding index.
To determine whether passage through START depends on completion of CDC45 function, we performed the reciprocal experiment. cdc45-1 cells were arrested at 11°C and released into α-factor-containing medium at 30°C. Fig. 3B shows that replication occurred within 60 min after release, suggesting that the passage through START does not depend on execution of CDC45 function. This finding is consistent with the observation that cdc45-arrested cells have budded, an event that requires passage through START. Together with results from the previous set of reciprocal shift experiments, these data indicate that CDC45 performs an essential replication function between START and DNA elongation.
Cdc45p Is Necessary for Triggering of the Pre-RC.
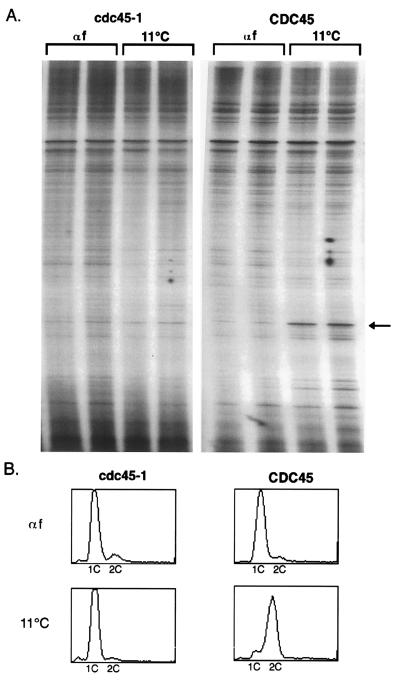
Current models (reviewed in refs. 24–26) suggest that initiation can be broken down into at least two steps: the assembly of pre-RCs at replication origins shortly after mitosis and the triggering of initiation during the G1/S phase transition (16). The experiments described above are consistent with CDC45 having a role in triggering initiation. To further explore this role, we examined the genomic footprint at the 2-micron origin of replication in cdc45-1 and CDC45 strains. In wild-type cells, changes in this footprint during the cell cycle are thought to reflect the assembly and disassembly of the pre-RC. During S, G2, and M phases, this footprint resembles that of purified ORC bound to the 2-micron origin and is most distinctly characterized by the presence of a hypersensitive site. ORC is thus thought to be bound to the origin during these phases of the cell cycle as part of a post-RC. In G1, loss of the hypersensitive site is the most dramatic change observed in the genomic footprint and is indicative of pre-RC assembly. Disassembly of the pre-RC is marked by reappearance of the post-RC with its signature hypersensitive site and tightly correlates with entry into S phase. Because of this correlation, the pre-RC to post-RC transition is thought to signal the triggering of initiation at origins (16) and was used by us to monitor the execution of the second initiation step.
CDC45 and cdc45-1 cells were arrested with α factor at the permissive temperature of 30°C. As expected, both strains arrested as unbudded cells with a 1C DNA content (Fig. 4B Upper). Moreover, in both strains the absence of the ORC-induced hypersensitive site in the genomic footprint of the 2-micron origin indicated the presence of the pre-RC (Fig. 4A, lanes αf). Cells were then released from the α-factor arrest at 11°C in the presence of nocodazole for 9 h. During this time CDC45 cells initiated and completed S phase, arresting at the nocodazole block with a 2C DNA content (Fig. 4B Lower). Genomic footprinting at this block reveals the presence of the hypersensitive site (Fig. 4A, lanes 11°C), consistent with the disassembly of the pre-RC during initiation. In contrast, cdc45-1 cells were unable to replicate and arrested as large-budded cells (confirming release from the α factor block) containing a 1C DNA content (Fig. 4B Lower). The genomic footprint at this arrest was virtually identical to the one observed at the α factor arrest (Fig. 4A, lanes 11°C), indicating that the pre-RC had not disassembled and was still present at the origin. The persistence of the pre-RC at the cdc45-1 block suggests that Cdc45p is needed to trigger initiation at replication origins.
Figure 4.
Genomic footprinting of cdc45-1 cells. CDC45 and cdc45-1 cells were arrested at START with α-factor-containing medium at 30°C (αf), and synchronously released at 11°C in the presence of nocodazole for 9 h (11°C). Each sample was processed in duplicate for genomic footprinting (A) and flow cytometry (B). The arrow indicates the hypersensitive site which is present at the 2-micron origin during S, G2, and M phases of the cell cycle.
Cdc45p Functions in the Same Step as Cdc7p/Dbf4p.
Mutations in CDC7 cause cells to arrest in late G1 phase at the last genetically defined point before S phase (11), suggesting that the kinase is required to trigger initiation. To determine the relative dependencies of CDC7 and CDC45, we performed reciprocal shift experiments with a cdc45-1 cdc7-4 double mutant strain. This experiment takes advantage of the fact that cdc45-1 is a cold-sensitive allele and cdc7-4 is a temperature-sensitive allele, allowing us to inactivate each gene independently of the other.
To determine whether CDC45 function is needed after a CDC7 block, we released cdc45-1 cdc7-4 double mutant cells from a cdc7-4 arrest at 38°C into medium at either 11°C, the restrictive temperature for cdc45-1, or 27°C, the permissive temperature for both mutants. The cells that were released into the permissive temperature replicated and attained a 2C DNA content within 60 min after release (Fig. 5B), whereas the cells placed at 11°C failed to replicate and remained with a 1C DNA content (Fig. 5A). This result suggests that execution of CDC45 function is dependent on CDC7.
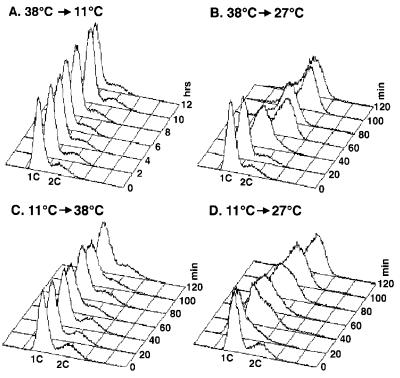
Figure 5.
CDC45 and CDC7 are dependent on one another for function. (A) A cdc45-1 cdc7-4 culture was arrested at 38°C, the restrictive temperature for cdc7-4. At 0 h, the culture was released into medium at 11°C, the restrictive temperature for cdc45-1. Samples were monitored by flow cytometry. (B) Cells were arrested in medium at 38°C. At 0 min, the culture was released at 27°C, the permissive temperature for both cdc45-1 and cdc7-4. (C) Cells were arrested in medium at 11°C. At 0 min, the culture was released at 38°C. (D) Cells were arrested in medium at 11°C. At 0 min, the culture was released at 27°C.
To determine whether the reverse might hold—i.e., that CDC7 function is dependent on execution of CDC45 function—we performed the reciprocal experiment. The cdc45-1 cdc7-4 cells were released from a cdc45 arrest at 11°C into media at either 38°C, the restrictive temperature for cdc7-4, or 27°C, the permissive temperature for both mutants. The cells that were released into the permissive temperature were able to replicate within 60 min (Fig. 5D), whereas the cells released to 38°C did not replicate even after 120 min (Fig. 5C). As additional controls we also repeated both temperature shift experiments with cdc45-1 and cdc7-4 single mutants to confirm that each is capable of releasing from their arrest when shifted to the other temperature extreme. Both cdc45-1 cells released from an 11°C arrest into 38°C and cdc7-4 cells released from a 38°C arrest to 11°C were able to replicate normally (data not shown). Thus, we conclude that Cdc45p and Cdc7p function interdependently—i.e., they are dependent on each other for function.
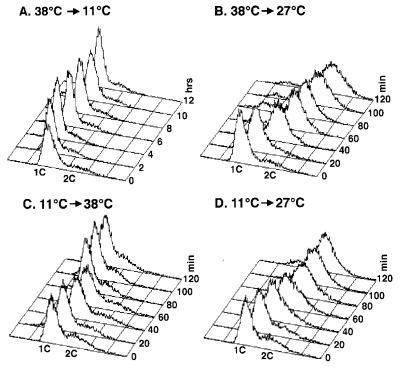
Given that Cdc7p kinase functions in association with a regulatory subunit, Dbf4p (12), we wondered whether Cdc45p and Dbf4p share a similar interdependent relationship. Hence, we performed the identical reciprocal shift experiments in cdc45-1 dbf4-1 double mutants. Double mutant cdc45-1 dbf4-1 cells released from a dbf4-1 arrest at 38°C into the permissive temperature for both mutations, 27°C, replicated and attained a 2C DNA content (Fig. 6B). In contrast, the same cells released into 11°C failed to replicate and retained a 1C DNA content (Fig. 6A). This result suggests that execution of CDC45 function is dependent on DBF4. Similarly, cdc45-1 dbf4-1 cells released from a cdc45 arrest at 11°C into the permissive temperature were able to replicate (Fig. 6D), whereas the cells did not replicate when released into 38°C (Fig. 6C). This finding indicates that execution of DBF4 function is dependent on CDC45. Thus, we conclude that, like CDC45 and CDC7, CDC45 and DBF4 function interdependently.
Figure 6.
CDC45 and DBF4 are dependent on one another for function. Experiments were performed identically to Fig. 5, except dbf4-1 was used instead of cdc7-4.
DISCUSSION
It has been demonstrated that CDC45 mRNA is periodically expressed during the cell cycle with levels peaking at the G1/S transition (32). This periodicity raises the possibility that CDC45 activity is regulated during the cell cycle through control of its protein levels. Although fluorescence microscopy studies have demonstrated that CDC45 protein persists in the nucleus throughout the cell cycle (29), a quantitative determination of protein levels had not been made. In this report, we show by immunoblot analysis that CDC45 protein is maintained at constant levels in cycling cells. Thus, if Cdc45p activity is regulated during the cell cycle, this regulation must occur by some mechanism besides modulation of protein levels or changes in subcellular localization. We note that it is formally possible that the epitope tag that we used to monitor Cdc45p levels affected the metabolism of the protein and thereby its steady-state levels. However, given that tagging the endogenous protein had no discernible effects on cell growth or replication, we anticipate that the levels of untagged Cdc45p will also prove to be constant once antibodies directed against Cdc45p itself become available.
We have shown that Cdc45p executes a function that is dependent on passage through START and is essential for replication elongation. This conclusion is consistent with a role for CDC45 in replication initiation. Previous efforts to order these events using the semi-restrictive temperature of 15°C were complicated by an inability to fully block DNA replication and achieve a reversible cdc45-1 arrest (31). Shifting cdc45-1 cells to 15°C delayed and prolonged but did not prevent S phase. We were able to demonstrate a strict dependence of replication on CDC45 function because cdc45-1 achieves a tight, reversible arrest at 11°C. Moreover, at this fully restrictive temperature cdc45-1 cells were able to synthesize DNA with nearly wild-type kinetics when released from a HU arrest. This latter result is similar to observations reported at 15°C (31) and, with the caveat that only a single allele of cdc45 has been examined, supports the notion that Cdc45p does not play an essential role in DNA elongation.
The events leading to the initiation of DNA replication can be divided into at least two stages: assembly of pre-RCs at origins shortly after mitosis and triggering of these complexes at the G1/S boundary (16). Our data suggest that when released from a START block at the restrictive temperature, cdc45-1 cells failed to enter S phase and arrested with the pre-RC still present at the 2-micron origin. In contrast, wild-type cells triggered initiation, disassembled the pre-RC, and replicated their DNA. Given the correlation between the initiation of DNA replication and the disappearance of pre-RCs (16), the persistence of pre-RCs in cdc45-1-arrested cells suggests that most 2-micron origins have not fired. This observation argues for a role of Cdc45p in triggering the pre-RC.
The persistence of the pre-replicative genomic footprint in cdc45-1 cells at the restrictive temperature raises the possibility that Cdc45p is not a component of the pre-RC. However, we cannot rule out Cdc45p being a part of the pre-RC because the incorporation of Cdc45p in the complex might not be affected by the cdc45-1 mutation. Furthermore, even if Cdc45p were a component of the pre-RC, its presence or absence in the complex may not affect the genomic footprint.
Cdc7p kinase, in association with its regulatory subunit Dbf4p (12), acts at the last genetically defined cell cycle step before entry into S phase (11) and is thought to help trigger initiation of DNA replication. Like the cdc45-1 mutant, cdc7 cells arrest in late G1 with the pre-RC still present at its origins (16). Our demonstration that CDC45 functions interdependently with both CDC7 and DBF4 further implicates CDC45 in the triggering of initiation and suggests that Cdc45p acts in concert with Cdc7p/Dbf4p kinase to carry out this event.
Genetic and biochemical studies suggests that the MCM family of proteins and ORC may also participate in the initiation events involving Cdc45p and Cdc7p/Dbf4p. First, cdc45-1 interacts genetically with mutants in several MCM and ORC components (31–33), and Cdc45p physically associates with at least one of the MCM proteins, Cdc46p/Mcm5p (29). Second, cdc7 and dbf4 deletion mutations can be suppressed by a mutation in CDC46/MCM5 (30). Third, Cdc7p kinase can phosphorylate several MCM proteins in vitro (B. K. Tye and A. Sugino, personal communication). These observations raise several possibilities of what Cdc45p could be doing at the G1/S boundary. One possibility is that it facilitates the activation of Cdc7p kinase in late G1. Another is based on the notion that the MCM proteins and ORC are components of the pre-RC (16, 23, 39) and that phosphorylation of these proteins by Cdc7p kinase contributes to activation of the pre-RC. In this scenario, Cdc45p may regulate the activity of Cdc7p by controlling access of the kinase to its substrates in the pre-RC. Finally, Cdc45p may itself be phosphorylated and activated by Cdc7p/Dbf4p to trigger the pre-RC. Elucidating the precise role of Cdc45p in triggering replication initiation will require further biochemical investigation.
Acknowledgments
We are grateful to David Botstein for the original cdc45-1 strain and Ross Okamura and Mary O’Riordan for technical assistance with flow cytometry and Southern blot analysis. We thank David Morgan, Alexander Johnson, Erin O’Shea, and Ira Herskowitz for critically reading the manuscript and members of the Li lab for helpful discussions. J.C.O. is supported by an Achievement Rewards for College Scientists Foundation graduate fellowship and by a National Institutes of Health training grant. C.S.D. is supported by a National Defense Science and Engineering Grant from the Department of Defense and by a National Institutes of Health training grant. J.J.L. is a Lucille P. Markey Scholar, a Searle Scholar, and a Rita Allen Foundation Scholar.
ABBREVIATIONS
- ORC
origin recognition complex
- MCM
minichromosome maintenance
- pre-RC
prereplicative complex
- post-RC
post replicative complex
- HU
hydroxyurea
- HA
hemagglutinin
References
- 1.Foss M, McNally F J, Laurenson P, Rine J. Science. 1993;262:1838–1844. doi: 10.1126/science.8266071. [DOI] [PubMed] [Google Scholar]
- 2.Fox C A, Loo S, Dillin A, Rine J. Genes Dev. 1995;9:911–924. doi: 10.1101/gad.9.8.911. [DOI] [PubMed] [Google Scholar]
- 3.Bell S P, Kobayashi R, Stillman B. Science. 1993;262:1844–1849. doi: 10.1126/science.8266072. [DOI] [PubMed] [Google Scholar]
- 4.Li J J, Herskowitz I. Science. 1993;262:1870–1874. doi: 10.1126/science.8266075. [DOI] [PubMed] [Google Scholar]
- 5.Loo S, Fox C A, Rine J, Kobayashi R, Stillman B, Bell S. Mol Biol Cell. 1995;6:741–756. doi: 10.1091/mbc.6.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Micklem G, Rowley A, Harwood J, Nasmyth K, Diffley J F. Nature (London) 1993;366:87–89. doi: 10.1038/366087a0. [DOI] [PubMed] [Google Scholar]
- 7.Dalton S, Whitbread L. Proc Natl Acad Sci USA. 1995;92:2514–2518. doi: 10.1073/pnas.92.7.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hennessy K M, Clark C D, Botstein D. Genes Dev. 1990;4:2252–2263. doi: 10.1101/gad.4.12b.2252. [DOI] [PubMed] [Google Scholar]
- 9.Whitbread L A, Dalton S. Gene. 1995;155:113–117. doi: 10.1016/0378-1119(94)00925-i. [DOI] [PubMed] [Google Scholar]
- 10.Yan H, Gibson S, Tye B K. Genes Dev. 1991;5:944–957. doi: 10.1101/gad.5.6.944. [DOI] [PubMed] [Google Scholar]
- 11.Hartwell L H. J Mol Biol. 1976;104:803–817. doi: 10.1016/0022-2836(76)90183-2. [DOI] [PubMed] [Google Scholar]
- 12.Sclafani R A, Jackson A L. Mol Microbiol. 1994;11:805–810. doi: 10.1111/j.1365-2958.1994.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 13.Jackson A L, Pahl P M, Harrison K, Rosamond J, Sclafani R A. Mol Cell Biol. 1993;13:2899–2908. doi: 10.1128/mcb.13.5.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowell S J, Romanowski P, Diffley J F. Science. 1994;265:1243–1246. doi: 10.1126/science.8066465. [DOI] [PubMed] [Google Scholar]
- 15.Schwob E, Bohm T, Mendenhall M D, Nasmyth K. Cell. 1994;79:233–244. doi: 10.1016/0092-8674(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 16.Diffley J F X, Cocker J H, Dowell S J, Rowley A. Cell. 1994;78:303–316. doi: 10.1016/0092-8674(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 17.Cocker J H, Piatti S, Santocanale C, Nasmyth K, Diffley J F. Nature (London) 1996;379:180–182. doi: 10.1038/379180a0. [DOI] [PubMed] [Google Scholar]
- 18.Detweiler C S, Li J J. J Cell Sci. 1997;110:753–763. doi: 10.1242/jcs.110.6.753. [DOI] [PubMed] [Google Scholar]
- 19.Kearsey S E, Maiorano D, Holmes E C, Todorov I T. BioEssays. 1996;18:183–190. doi: 10.1002/bies.950180305. [DOI] [PubMed] [Google Scholar]
- 20.Chong J P, Thommes P, Blow J J. Trends Biochem Sci. 1996;21:102–106. [PubMed] [Google Scholar]
- 21.Tye B-K. Trends Cell Biol. 1994;4:160–166. doi: 10.1016/0962-8924(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 22.Yan H, Merchant A M, Tye B K. Genes Dev. 1993;7:2149–2160. doi: 10.1101/gad.7.11.2149. [DOI] [PubMed] [Google Scholar]
- 23.Coleman T R, Carpenter P B, Dunphy W G. Cell. 1996;87:53–63. doi: 10.1016/s0092-8674(00)81322-7. [DOI] [PubMed] [Google Scholar]
- 24.Muzi-Falconi M, Brown G W, Kelly T J. Curr Biol. 1996;6:229–233. doi: 10.1016/s0960-9822(02)00464-5. [DOI] [PubMed] [Google Scholar]
- 25.Wang T A, Li J J. Curr Opin Cell Biol. 1995;7:414–420. doi: 10.1016/0955-0674(95)80098-0. [DOI] [PubMed] [Google Scholar]
- 26.Diffley J F. Genes Dev. 1996;10:2819–2830. doi: 10.1101/gad.10.22.2819. [DOI] [PubMed] [Google Scholar]
- 27.Epstein C B, Cross F R. Genes Dev. 1992;6:1695–1706. doi: 10.1101/gad.6.9.1695. [DOI] [PubMed] [Google Scholar]
- 28.Schwob E, Nasmyth K. Genes Dev. 1993;7:1160–1175. doi: 10.1101/gad.7.7a.1160. [DOI] [PubMed] [Google Scholar]
- 29.Hopwood B, Dalton S. Proc Natl Acad Sci USA. 1996;93:12309–12314. doi: 10.1073/pnas.93.22.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardy C F, Dryga O, Seematter S, Pahl P M, Sclafani R A. Proc Natl Acad Sci USA. 1997;94:3151–3155. doi: 10.1073/pnas.94.7.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou L, Mitchell J, Stillman B. Mol Cell Biol. 1997;17:553–563. doi: 10.1128/mcb.17.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hardy C F. Gene. 1997;187:239–246. doi: 10.1016/s0378-1119(96)00761-5. [DOI] [PubMed] [Google Scholar]
- 33.Hennessy K M, Lee A, Chen E, Botstein D. Genes Dev. 1991;5:958–969. doi: 10.1101/gad.5.6.958. [DOI] [PubMed] [Google Scholar]
- 34.Guthrie C, Fink G R. Methods Enzymol. 1991;194:281–301. [Google Scholar]
- 35.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1997. [Google Scholar]
- 36.Tyers M, Tokiwa G, Nash R, Futcher B. EMBO J. 1992;11:1773–1784. doi: 10.1002/j.1460-2075.1992.tb05229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diffley J F X, Cocker J H. Nature (London) 1992;357:169–172. doi: 10.1038/357169a0. [DOI] [PubMed] [Google Scholar]
- 38.Huibregtse J M, Engelke D R. Methods Enzymol. 1991;194:550–562. doi: 10.1016/0076-6879(91)94042-b. [DOI] [PubMed] [Google Scholar]
- 39.Donovan S, Harwood J, Drury L S, Diffley J F. Proc Natl Acad Sci USA. 1997;94:5611–5616. doi: 10.1073/pnas.94.11.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]