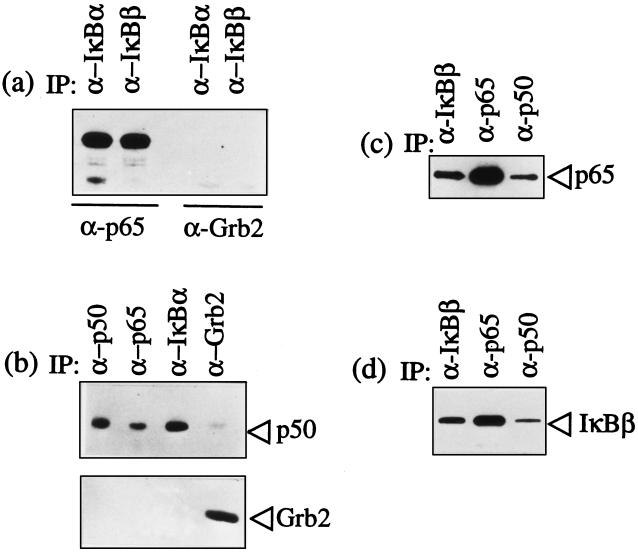
Figure 3.
T. parva infection does not abrogate IκBα and IκBβ binding to p65 RelA and p50/p65 RelA heterodimers. The specific antibodies used in immunoprecipitation are designated above each lane in the figures. The immunoprecipitated complexes were then analyzed using immunoblots to identify coprecipitated components. (a) IκBα and IκBβ bind p65 RelA. Immunoblot analysis of IκBα and IκBβ immunoprecipitates with anti-p65 RelA antibodies detected bound p65 RelA. The immunoblot with antibody against Grb2 was used as a negative control for nonspecific complex formation. (b) p50 is bound to both IκBα and p65 RelA. Immunoblot analysis of p65 and IκBα immunoprecipitates with anti-p50 antibodies detected bound p50. The p50 and Grb2 immunoprecipitates were included as positive and negative controls, respectively. (c) p65 RelA is bound to both IκBβ and p50. Immunoblot analysis of p50 and IκBβ immunoprecipitates with anti-p65 antibodies detected bound p65. The p65 immunoprecipitate was included as a positive control. (d) IκBβ is bound to both p50 and p65 RelA. Immunoblot analysis of p50 and p65 RelA immunoprecipitates with anti-IκBβ antibodies detected bound IκBβ. The IκBβ immunoprecipitate was included as a positive control.