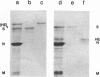
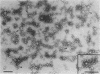
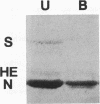
Abstract
The S protein of bovine coronavirus (BCV) has been isolated from the viral membrane and purified by gradient centrifugation. Purified S protein was identified as a viral hemagglutinin. Inactivation of the cellular receptors by sialate 9-O-acetylesterase and generation of receptors by sialylation of erythrocytes with N-acetyl-9-O-acetylneuraminic acid (Neu5,9Ac2) indicate that S protein recognizes 9-O-acetylated sialic acid as a receptor determinant as has been shown previously for intact virions. The second glycoprotein of BCV, HE, which has been thought previously to be responsible for the hemagglutinating activity of BCV, is a less efficient hemagglutinin; it agglutinates mouse and rat erythrocytes, but in contrast to S protein, it is unable to agglutinate chicken erythrocytes, which contain a lower level of Neu5,9Ac2 on their surface. S protein is proposed to be responsible for the primary attachment of virus to cell surface. S protein is proposed to be responsible for the primary attachement of virus to cell surface receptors. The potential of S protein as a probe for the detection of Neu5,9Ac2-containing glycoconjugates is demonstrated.
Full text
PDF


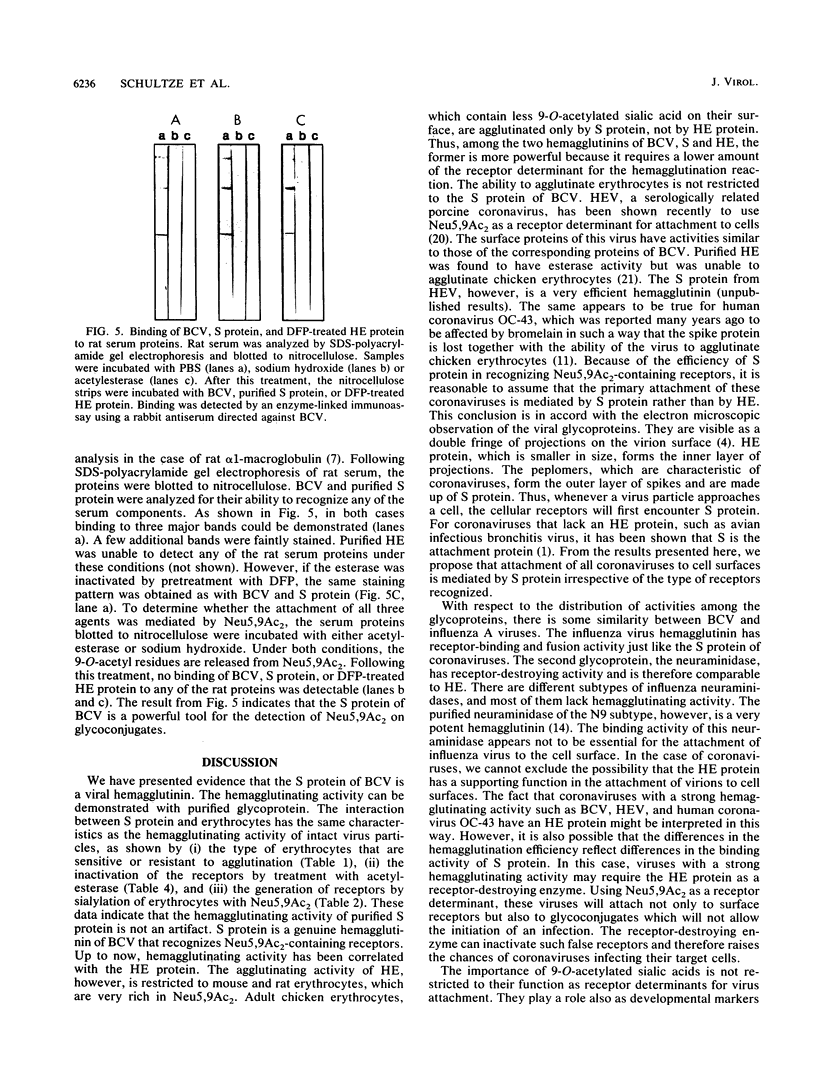

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavanagh D., Davis P. J. Coronavirus IBV: removal of spike glycopolypeptide S1 by urea abolishes infectivity and haemagglutination but not attachment to cells. J Gen Virol. 1986 Jul;67(Pt 7):1443–1448. doi: 10.1099/0022-1317-67-7-1443. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Varki A. P., Varki N. M., Stallcup W. B., Levine J., Reisfeld R. A. A monoclonal antibody recognizes an O-acylated sialic acid in a human melanoma-associated ganglioside. J Biol Chem. 1984 Jun 25;259(12):7453–7459. [PubMed] [Google Scholar]
- Compans R. W., Klenk H. D., Caliguiri L. A., Choppin P. W. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970 Dec;42(4):880–889. doi: 10.1016/0042-6822(70)90337-5. [DOI] [PubMed] [Google Scholar]
- Dea S., Garzon S., Tijssen P. Identification and location of the structural glycoproteins of a tissue culture-adapted turkey enteric coronavirus. Arch Virol. 1989;106(3-4):221–237. doi: 10.1007/BF01313955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formanowski F., Meier-Ewert H. Isolation of the influenza C virus glycoprotein in a soluble form by bromelain digestion. Virus Res. 1988 May;10(2-3):177–191. doi: 10.1016/0168-1702(88)90014-7. [DOI] [PubMed] [Google Scholar]
- Herrler G., Dürkop I., Becht H., Klenk H. D. The glycoprotein of influenza C virus is the haemagglutinin, esterase and fusion factor. J Gen Virol. 1988 Apr;69(Pt 4):839–846. doi: 10.1099/0022-1317-69-4-839. [DOI] [PubMed] [Google Scholar]
- Herrler G., Geyer R., Müller H. P., Stirm S., Klenk H. D. Rat alpha 1 macroglobulin inhibits hemagglutination by influenza C virus. Virus Res. 1985 Mar;2(2):183–192. doi: 10.1016/0168-1702(85)90248-5. [DOI] [PubMed] [Google Scholar]
- Herrler G., Reuter G., Rott R., Klenk H. D., Schauer R. N-acetyl-9-O-acetylneuraminic acid, the receptor determinant for influenza C virus, is a differentiation marker on chicken erythrocytes. Biol Chem Hoppe Seyler. 1987 May;368(5):451–454. doi: 10.1515/bchm3.1987.368.1.451. [DOI] [PubMed] [Google Scholar]
- Herrler G., Rott R., Klenk H. D., Müller H. P., Shukla A. K., Schauer R. The receptor-destroying enzyme of influenza C virus is neuraminate-O-acetylesterase. EMBO J. 1985 Jun;4(6):1503–1506. doi: 10.1002/j.1460-2075.1985.tb03809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrler G., Rott R., Klenk H. D. Neuraminic acid is involved in the binding of influenza C virus to erythrocytes. Virology. 1985 Feb;141(1):144–147. doi: 10.1016/0042-6822(85)90190-4. [DOI] [PubMed] [Google Scholar]
- Hierholzer J. C., Palmer E. L., Whitfield S. G., Kaye H. S., Dowdle W. R. Protein composition of coronavirus OC 43. Virology. 1972 May;48(2):516–527. doi: 10.1016/0042-6822(72)90062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B., Potts B. J., Brian D. A. Bovine coronavirus hemagglutinin protein. Virus Res. 1985 Feb;2(1):53–59. doi: 10.1016/0168-1702(85)90059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Colman P. M., Webster R. G., Hinshaw V. S., Air G. M. Influenza virus neuraminidase with hemagglutinin activity. Virology. 1984 Sep;137(2):314–323. doi: 10.1016/0042-6822(84)90223-x. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Valentine R. C. Morphology of the isolated hemagglutinin and neuraminidase subunits of influenza virus. Virology. 1969 May;38(1):105–119. doi: 10.1016/0042-6822(69)90132-9. [DOI] [PubMed] [Google Scholar]
- Parker M. D., Cox G. J., Deregt D., Fitzpatrick D. R., Babiuk L. A. Cloning and in vitro expression of the gene for the E3 haemagglutinin glycoprotein of bovine coronavirus. J Gen Virol. 1989 Jan;70(Pt 1):155–164. doi: 10.1099/0022-1317-70-1-155. [DOI] [PubMed] [Google Scholar]
- Paulson J. C., Rogers G. N. Resialylated erythrocytes for assessment of the specificity of sialyloligosaccharide binding proteins. Methods Enzymol. 1987;138:162–168. doi: 10.1016/0076-6879(87)38013-9. [DOI] [PubMed] [Google Scholar]
- Rogers G. N., Herrler G., Paulson J. C., Klenk H. D. Influenza C virus uses 9-O-acetyl-N-acetylneuraminic acid as a high affinity receptor determinant for attachment to cells. J Biol Chem. 1986 May 5;261(13):5947–5951. [PubMed] [Google Scholar]
- Schultze B., Gross H. J., Brossmer R., Klenk H. D., Herrler G. Hemagglutinating encephalomyelitis virus attaches to N-acetyl-9-O-acetylneuraminic acid-containing receptors on erythrocytes: comparison with bovine coronavirus and influenza C virus. Virus Res. 1990 Jun;16(2):185–194. doi: 10.1016/0168-1702(90)90022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze B., Wahn K., Klenk H. D., Herrler G. Isolated HE-protein from hemagglutinating encephalomyelitis virus and bovine coronavirus has receptor-destroying and receptor-binding activity. Virology. 1991 Jan;180(1):221–228. doi: 10.1016/0042-6822(91)90026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla A. K., Schauer R. Fluorimetric determination of unsubstituted and 9(8)-O-acetylated sialic acids in erythrocyte membranes. Hoppe Seylers Z Physiol Chem. 1982 Mar;363(3):255–262. doi: 10.1515/bchm2.1982.363.1.255. [DOI] [PubMed] [Google Scholar]
- Vlasak R., Krystal M., Nacht M., Palese P. The influenza C virus glycoprotein (HE) exhibits receptor-binding (hemagglutinin) and receptor-destroying (esterase) activities. Virology. 1987 Oct;160(2):419–425. doi: 10.1016/0042-6822(87)90013-4. [DOI] [PubMed] [Google Scholar]
- Vlasak R., Luytjes W., Leider J., Spaan W., Palese P. The E3 protein of bovine coronavirus is a receptor-destroying enzyme with acetylesterase activity. J Virol. 1988 Dec;62(12):4686–4690. doi: 10.1128/jvi.62.12.4686-4690.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasak R., Luytjes W., Spaan W., Palese P. Human and bovine coronaviruses recognize sialic acid-containing receptors similar to those of influenza C viruses. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4526–4529. doi: 10.1073/pnas.85.12.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]