Abstract
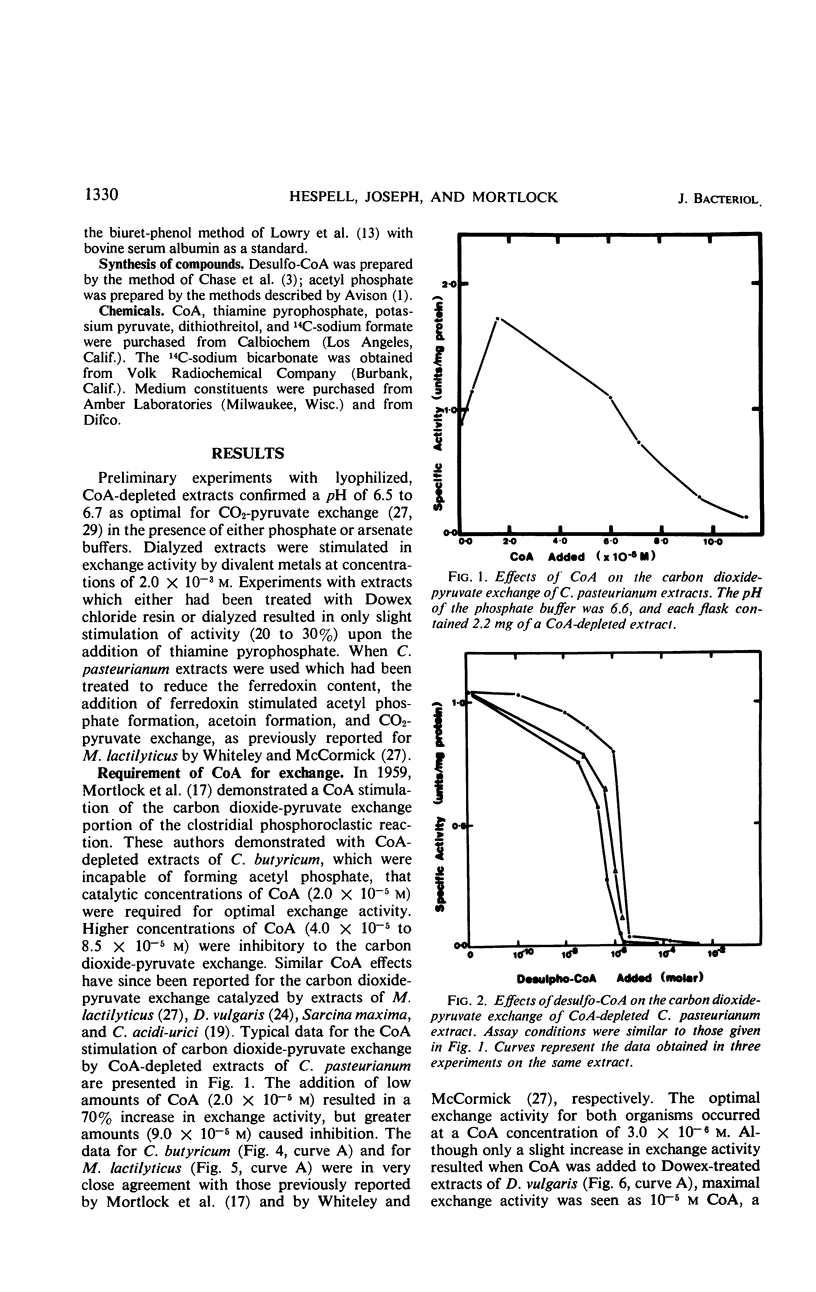
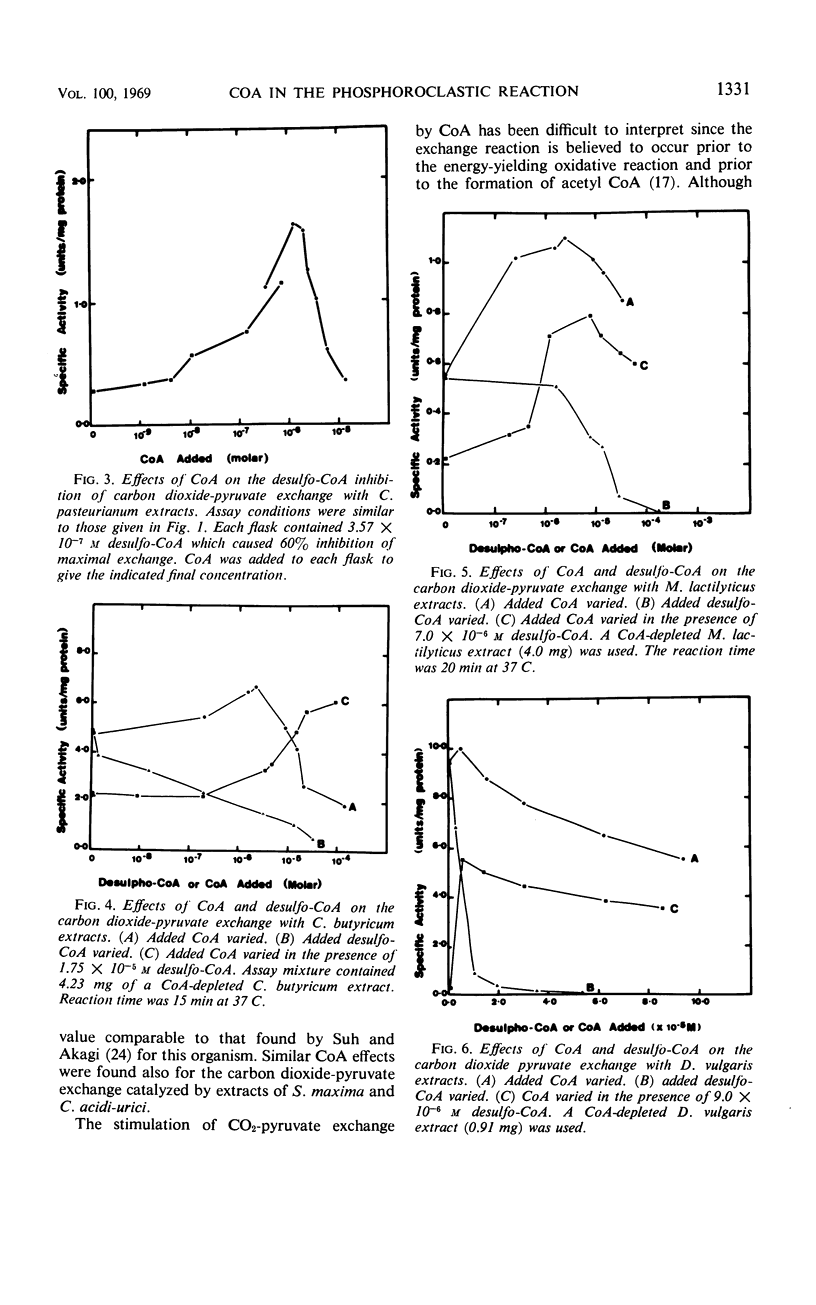
Various bacteria which degrade pyruvate by the phosphoroclastic reaction were examined with respect to the role of coenzyme A (CoA) in this reaction. The strictly anaerobic bacteria, which cleave pyruvate by the phosphoroclastic reaction characteristic of Clostridia, required catalytic levels of CoA for the CO2-pyruvate exchange and acetoin-forming portions of the phosphoroclastic reaction. These reactions were reversibly inhibited by the CoA analogue, desulfo-CoA. In contrast, using cell-free extracts of bacteria which degrade pyruvate by the coliform phosphoroclastic reaction (pyruvate formate-lyase), no requirement for CoA could be observed for the formate-pyruvate exchange reaction. It is suggested that CoA serves a regulatory function in the early portion of the clostridal type of phosphoroclastic reaction.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggins D. R., Dilworth M. J. Control of pyruvate phosphoroclastic activity in extracts of Clostridium pasteurianum by ADP and acetyl phosphate. Biochim Biophys Acta. 1968 Mar 11;156(2):285–296. doi: 10.1016/0304-4165(68)90257-2. [DOI] [PubMed] [Google Scholar]
- Chase J. F., Middleton B., Tubbs P. K. A coenzyme A analogue, desulpho-coA; preparation and effects on various enzymes. Biochem Biophys Res Commun. 1966 Apr 19;23(2):208–213. doi: 10.1016/0006-291x(66)90529-8. [DOI] [PubMed] [Google Scholar]
- Chase T., Jr, Rabinowitz J. C. Role of pyruvate and S-adenosylmethioine in activating the pyruvate formate-lyase of Escherichia coli. J Bacteriol. 1968 Oct;96(4):1065–1078. doi: 10.1128/jb.96.4.1065-1078.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang L. F., Collins E. B. Biosynthesis of diacetyl in bacteria and yeast. J Bacteriol. 1968 Jun;95(6):2083–2089. doi: 10.1128/jb.95.6.2083-2089.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNI E. Mechanisms of formation of acetoin by bacteria. J Biol Chem. 1952 Apr;195(2):715–726. [PubMed] [Google Scholar]
- JUNI E. Mechanisms of the formation of acetoin by yeast and mammalian tissue. J Biol Chem. 1952 Apr;195(2):727–734. [PubMed] [Google Scholar]
- Kupfer D. G., Canale-Parola E. Pyruvate metabolism in Sarcina maxima. J Bacteriol. 1967 Oct;94(4):984–990. doi: 10.1128/jb.94.4.984-990.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindmark D. G., Paolella P., Wood N. P. The pyruvate formate-lyase system of Streptococcus faecalis. I. Purification and properties of the formate-pyruvate exchange enzyme. J Biol Chem. 1969 Jul 10;244(13):3605–3612. [PubMed] [Google Scholar]
- MORTLOCK R. P., VALENTINE R. C., WOLFE R. S. Carbon dioxide activation in the pyruvate clastic system of Clostridium butyricum. J Biol Chem. 1959 Jul;234(7):1653–1656. [PubMed] [Google Scholar]
- McCormick N. G., Ordal E. J., Whiteley H. R. DEGRADATION OF PYRUVATE BY MICROCOCCUS LACTILYTICUS I. : General Properties of the Formate-Exchange Reaction. J Bacteriol. 1962 Apr;83(4):887–898. doi: 10.1128/jb.83.4.887-898.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSTER M. O., WOOD N. P. FORMATE--PYRUVATE EXCHANGE REACTION IN STREPTOCOCCUS FAECALIS. II. REACTION CONDITIONS FOR CELL EXTRACTS. J Bacteriol. 1964 Jan;87:104–113. doi: 10.1128/jb.87.1.104-113.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAEBURN S., RABINOWITZ J. C. PYRUVATE SYNTHESIS BY A PARTIALLY PURIFIED ENZYME FROM CLOSTRIDIUM ACIDI-URICI. Biochem Biophys Res Commun. 1965 Feb 3;18:303–307. doi: 10.1016/0006-291x(65)90703-5. [DOI] [PubMed] [Google Scholar]
- STRECKER H. J. Formate fixation in pyruvate by Escherichia coli. J Biol Chem. 1951 Apr;189(2):815–830. [PubMed] [Google Scholar]
- Speckman R. A., Collins E. B. Diacetyl biosynthesis in Streptococcus diacetilactis and Leuconostoc citrovorum. J Bacteriol. 1968 Jan;95(1):174–180. doi: 10.1128/jb.95.1.174-180.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Still J. L. Pyruvic dehydrogenase of Bacterium coli. Biochem J. 1941 Mar;35(3):380–389. doi: 10.1042/bj0350380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh B., Akagi J. M. Pyruvate-carbon dioxide exchange reaction of Desulfovibrio desulfuricans. J Bacteriol. 1966 Jun;91(6):2281–2285. doi: 10.1128/jb.91.6.2281-2285.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITELEY H. R., McCORMICK N. G. Degradation of pyruvate by Micrococcus lactilyticus. III. Properties and cofactor requirements of the carbon dioxide-exchange reaction. J Bacteriol. 1963 Feb;85:382–393. doi: 10.1128/jb.85.2.382-393.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFE R. S., O'KANE D. J. Cofactors of the carbon dioxide exchange reaction of Clostridium butyricum. J Biol Chem. 1955 Aug;215(2):637–643. [PubMed] [Google Scholar]
- WOLFE R. S., O'KANE D. J. Cofactors of the phosphoroclastic reaction of Clostridium butyricum. J Biol Chem. 1953 Dec;205(2):755–765. [PubMed] [Google Scholar]
- WOOD N. P., O'KANE D. J. FORMATE-PYRUVATE EXCHANGE REACTION IN STREPTOCOCCUS FAECALIS. I. FACTOR REQUIREMENT FOR INTACT CELLS. J Bacteriol. 1964 Jan;87:97–103. doi: 10.1128/jb.87.1.97-103.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]


