Table 3.
Selective Protection of (E)-1,5-anti-diols
| entry | substrate (R = H) |
producta (R = SiEt3) |
yieldb,c | allylic: homoallylic |
|---|---|---|---|---|
| 1 | 19 | 66% | 95:5 | |
| 2 | 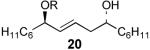 |
21 | 66% | >97:3 |
| 3 | 23 | 79% | >97:3 | |
| 4 | 25 | 44% | 84:16 | |
| 5 |  |
27 | 58% | 77:22 |
Reactions were performed by treating a solution of the 1,5-diol (1 equiv), imidazole (1.15 equiv), and DMAP (0.05 equiv) in CH2Cl2-DMF (1:1, 0.1 M) at −78 °C with TES-Cl (1.1 equiv)
Combined yield of mono-TES esters (allylic and homoallylic)
bis-TES ethers were obtained as follows: entry 1, 20%; entry 2, 2%, entry 3, 2%, entry 4, 16%, entry 5, 16%.
