Abstract
Motility of cancer cells plays a critical role in tumor metastasis, and as such is a target for intervention. The motility of malignant Calu-1 human lung epithelial carcinoma cells is up-regulated when placed on a human umbilical vein endothelial cell monolayer, while that of non-malignant L132 human lung epithelial cells is not. To dissect the factor(s) causing such differential behaviors, the motile responses of both cell lines to endothelial cell factors – secreted to the media, on the endothelial cell surface, and secreted to the extracellular matrix – and to individual extracellular matrix proteins were compared. Cell motility was quantified by tracking the cell movement on a surface with time-lapse video microscopy, which was analyzed with the persistent random walk model of motility. None of the factors tested had a remarkable effect on L132 cell motility, but the Calu-1 cell motility was significantly up-regulated by endothelial cell extracellular matrix and by laminin, fibronectin, collagen I and collagen VI individually. Flow cytometry analysis revealed significantly higher expression levels of integrin subunits β1, α2, α3 and α6, which are known receptors for these extracellular matrix proteins, on the Calu-1 than L132 cells, implicating a role of these integrins in the observed motile behaviors of these cell lines.
Keywords: tumor cell motility, persistent random walk, extracellular matrix, integrin
Introduction
The blood-borne dissemination of tumor cells is a complex process involving a cascade of events. It includes initial growth of a primary tumor, angiogenesis, intravasation from the tumor to the blood stream, extravasation from the blood stream at a distant site, and the growth of secondary tumors. Motility of tumor cells is an integral part of this cascade, as it is required for both intravasation into the blood stream from the primary tumor and extravasation out of the blood stream into the surrounding tissue.
It is generally believed that, like all normal cells, tumor cells are equipped with the necessary motile machinery for migration. However, this machinery must be turned on for tumor cells to acquire the needed motility for metastasis. Three classes of factors are known to upregulate tumor cell motility: 1. insulin-like growth factors, 2. cytokines, and 3. extracellular matrix (ECM) proteins. These molecules can be either soluble or substrate-bound, and can be secreted by either tumor cells or host cells. Soluble chemotactic or chemokinetic factors include fragments of tissue and basement membrane proteins released by proteases, autocrine motility factors, and polypeptide growth factors20. Substrate-bound factors include receptors present on the surface of endothelial cells and components of their ECM.
The vascular endothelium is an anatomic barrier for the transport of blood components, including cellular elements, across the vessel wall. It also expresses factors that could mediate the leukocyte attachment to and migration across the blood vessel wall. Published work on tumor cell invasion has demonstrated that cell lines known to be malignant and/or invasive in vivo often have a greater ability to penetrate barriers such as cell monolayers in vitro3,17,21. Previously, we showed by a modified Boyden Chamber assay that compared to its non-malignant counterpart L132 cells, the malignant tumor cell line Calu-1 was more able to invade endothelial monolayer and basement membrane barriers19. Here we present in vitro evidence that the interaction between tumor cells and endothelial cells results in a differential upregulation of tumor cell motility, which may be relevant to their ability to migrate across the barrier layers. Our data are consistent with the hypothesis that tumor cells, via increased expression of cell surface receptors, are capable of opportunistic use of molecules secreted by the endothelial cells to upregulate their motility.
This hypothesis was tested in a two-part process. To narrow down possible receptor types to investigate, we first determined if any specific category of endothelial cell factors had a greater effect on tumor cell motility than others. This was done by comparing the motile response of the Calu-1 cells and the L132 cells to the factors from Human Umbilical Vein Endothelial Cells (HUVECs). These included: 1. Constitutively secreted soluble factors, 2. Soluble factors induced by tumor cells, and 3. Constitutively secreted extracellular matrix proteins. The cells’ motile responses to purified ECM proteins were also tested. Planary motile activities of malignant and non-malignant cells were observed using computer controlled time-lapse microscopy, quantified using digital image acquisition, and analyzed using a persistent random walk model.
It was found that none of the factors tested had a remarkable up-regulatory effect on L132 cell motility, but the Calu-1 cell motility was significantly up-regulated by the HUVEC secreted ECM, and was up-regulated differentially by the individual ECM proteins tested. These results allowed us to focus on the primary receptors for the extracellular matrix proteins: the integrins. Flow cytometry analysis revealed that the Calu-1 cells express higher levels of integrin subunits α2, α3, α6, and β1 than L132 cells. The correlation between observed motile behavior and integrin expression levels suggests a possible mechanism for the differences in motility of the two cell types.
Materials and Methods
Cell Culture
HUVECs, obtained from Clonetics (Walkersville, MD), were cultured according to standard practices in Endothelial Cell Basal Medium-2 (EBM-2) media (Clonetics) supplemented with 2% Fetal Bovine Serum (FBS), 0.04% hydrocortisone, 0.4% Human Fibroblast Growth Factor (hFGF), and 0.1% Vascular Endothelial Cell Growth Factor (VEGF), R3- Insulin-like Growth Factor (IGF-1), ascorbic acid, Human Epidermal Growth Factor (hEGF), GA-1000, and heparin. Human lung epidermoid carcinoma Calu-1 cells and human embryonic lung epithelial L132 cells obtained from American Type Culture Collection (Rockville, MD) were cultured in Dulbecco's Modified Eagle's Medium (DMEM, Sigma Chemicals, St. Louis, MO) supplemented with 10% FBS.
Phosphate Buffered Saline (PBS), Disodium Ethylenediamine Tetraacetate (EDTA), Bovine Serum Albumin (BSA), Sodium Dodecyl Sulfate (SDS), and other cell culture reagents were obtained from Sigma. FBS was obtained from Gibco BRL Life Technologies (Grand Island, NY). HUVEC- and tumor cell-conditioned EBM-2 media were obtained by collecting media after 24 to 48 hours of exposure to the cells and filtering through a 0.2 μm filter. Co-cultured-conditioned media were obtained by placing Calu-1 or L132 cells in the bottom of a well below a filter on which a confluent monolayer of HUVECs was grown, allowing contact between the two cell types via the media only. After 24 hours, the media was collected and filtered as above.
The confluent monolayers of HUVECs used for motility assays were grown in 24-well plates. HUVECs were used at passage three to six.
Antibodies
Monoclonal antibodies (mAbs, * denotes functional blocking) directed against α1 (FB12), α2 (P1E6*), α3 (P1B5*), αv (P3G8), and β4 (3E1) were from Chemicon International, Inc. (Temecula, CA). The anti-α4 (P4G9) and anti-β1 (P4C10*) mAbs were from Gibco, anti-α5 (VC5) and anti-β3 (V1PL2) mAbs were from Pharmingen (San Diego, CA), and anti-α6 mAb (GoH3*) was from Beckman Coulter (Fullerton, CA). The irrelevant control mAb (X63) was a generous gift of Dr. Periasamy Selvaraj of Emory University (Atlanta, GA).
Determination of Surface Expression of Integrins
Cells were stained for flow cytometry (fluorescence activated cell sorting, FACS) using a standard two-step protocol. Washed cells (2.5 − 5 × 105 / sample) were placed in the wells of a V-bottom 96-well microtiter plate which had been pre-coated with FACS buffer (RPMI with 5 mM EDTA, 1% FBS, and 0.02% sodium azide) for 1 hour at room temperature. The cells were then incubated for 30 minutes with primary antibody (mouse anti-human integrin) at 4°C while being agitated with a shaker. The cells were then washed three times with the FACS buffer, followed by another 30 minute incubation with a saturating concentration of FITC-conjugated anti-mouse IgG secondary antibody (Sigma), carried out on a shaker at 4°C. After being washed three times with FACS buffer the cells were fixed with 3.7% formaldehyde in PBS and stored at 4°C in wrapped foil until being analyzed, usually within 24 hours.
The stained cells were analyzed using a FACScan flow cytometer (Becton-Dickinson, Bedford, MA) and Cellquest software. Generally, 40,000 events were recorded per sample. In order to calculate the molecules of equivalent soluble fluorophore (MESF) values of the cells, the standard beads (Q25, Flow Cytometry Standards, San Juan) were also analyzed.
Data are reported as the mean fluorescence level of the events recorded from each sample. This mean is standardized to MESF using a conversion formula generated using the mean fluorescence levels recorded for the beads and their known MESF values provided by the manufacturer.
Extracellular Matrix Protein Preparation and Coating
To obtain HUVEC ECM, confluent HUVEC monolayers grown on 24-well plates were removed by incubation with 5 mM EDTA in PBS for 20−60 minutes. The remaining ECM was washed two times with plain DMEM.
Collagen I, collagen IV, and gelatin were obtained from Sigma. Vitronectin was obtained from Gibco. Fibronectin, laminin, and matrigel were obtained from Becton Dickinson. To coat wells with isolated ECM proteins, 250 μL of each protein (10 μg mL−1) in PBS (except for Matrigel, the concentration of which was 250 μg mL−1) was placed in individual wells of a 24-well plate and kept at 37°C overnight. Prior to use, excess solution was aspirated, and the coated plate was washed two times with PBS.
Motility Assay
A computer-controlled microscope stage and environmental chamber system was constructed in-house to acquire time-lapse sequences of microscopic images for analysis of cell motility under multiple conditions with needed replications. Similar systems are commercially available and have been used by others for long-term motility assays6, 18, 30. A detailed description for the construction and testing of our system can be found in Ref. 31. Briefly, computer control of the system was achieved with a four-axis Programmable Multi-Axis Control board (PMAC-Lite) from Delta Tau Data Systems (Northridge, CA) placed in a personal computer. An Olympus IMT-2 inverted microscope (Lake Success, N.Y.) was equipped with a Scan IM 100×100 stepper motor driven stage (Marzhauser Wetzlar Gmbh and Co., KG, Germany). Using the controller board, the stage was programmed to repeatedly scan to three preset positions of interest in each well in a 24-well plate, with a cycle time of ten minutes. An image of the field of view at each position was captured using a camera (Microimage Video Systems, Boyertown, PA) mounted to the microscope and linked to an image grabber board (Matrox International, Quebec, Canada) in the computer. The controller board in the computer was also used to trigger the image board to grab images.
A Plexiglas chamber was constructed around the microscope stage area, and the controller board in the computer was used to maintain a temperature of 37°C and a 95% air/5% CO2 mixture in this chamber.
7 × 104 test cells (Calu-1 or L132) in 1 mL of the appropriate media were added to each well of a 24-well plate on which various substrates had been prepared, and allowed to settle to the bottom surface for one hour. This corresponds to a seeding density of 3.68 × 104 cells/cm2, which allowed a sufficient number of cells for reliable statistical analysis yet minimized cell-cell contact. The fields of view to be observed (each contained 8−12 cells to be tracked) were determined and programmed. The stage then repeatedly scanned through all of the preset positions, and an image of each field was captured once every 10 minutes for 6 hours. In a set of experimental conditions, up to nine conditions and a replication of each condition were placed in one 24-well plate. All conditions and replications in a plate were performed with the same batches of cells. If a set of experimental conditions was too large, it was split into two plates and performed on consecutive days. Each set of experiments was also repeated at least once to ensure the reproducibility of the results.
The images of each field of view were saved and sorted on-line, and were compiled into a ‘time-lapse movie’ and analyzed off-line. The centroid of each cell was determined by visual inspection and recorded using Personal AVI Editor software (FlickerFree Multimedia Products, Denmark). Validation experiments show that the position accuracy of the scanning stage system is ± 1 μm31.
Cell centroid data were used to calculate the mean square distance (〈d2(t)〉) traveled as a function of the time interval t for each cell using overlapping time intervals9. For each condition studied, 55 to 60 cells were tracked and the mean square distance were calculated for 10 time intervals (t = 10, 20, ... , 100 minutes), yielding 550 to 600 data points per condition. The 〈d2(t)〉 vs. t data were then fit to the persistent random walk model1, 11,
| (1) |
where P is the directional persistence time, a characteristic time scale during which a cell migrates without significantly changing direction, and μ is a random motility coefficient that measures the level of dispersion of the cell tracks and is analogous to a molecular diffusion coefficient. Thus this model quantifies the statistical behavior of migrating cells whose motions persist in the same direction over short time periods but have significantly random elements over longer time periods. The nonlinear ordinary least squares curve fitting to the mean square distance data was done by KaleidaGraph™ (Synergy Software, Reading, PA), which uses the Levenberg-Marquandt algorithm of chi-squares error minimization to compute μ and P as well as the s.e.m. (standard error of the mean) of each. Different formulations of the persistent random walk model can be used with alternative parameters: the root mean squared speed S and the persistence length L (= SP) or any two of μ or S, and P or L. To provide a visual of the behavior modeled, cell tracks of three different levels of motility are shown as “dispersion plots” in Figure 1 where the parameter values are indicated. Preliminary experiments were performed to examine the sensitivity of the four parameters to changes in the migratory behaviors of the cells being studied. As exemplified in Figure 1, S was found to be the least reflective of the amount of dispersion observed in the present work. It can also be seen that μ, P, and L appear to be similarly indicative of the cell's migratory behaviors. This figure is representative of all the cases examined in the present study, including both Calu-1 and L132 cells migrating on a variety of substrates. Therefore, μ was chosen as the most revealing parameter to compare the differential cell motility.
Figure 1.
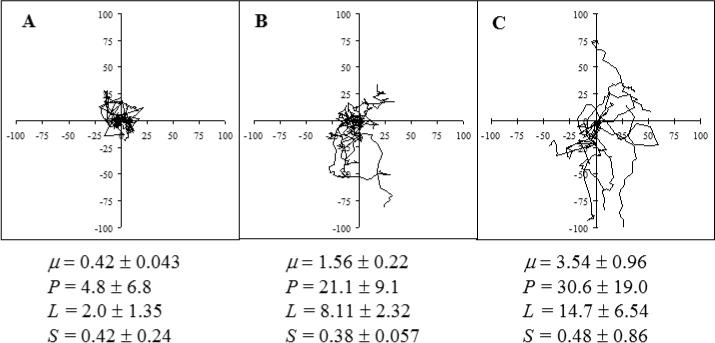
Dispersion Plots and Motility Parameters. Tracks of ten cells each from three different conditions of varying degrees of motility were superimposed with the origin of each track placed at the graph origins. Motility parameters (mean ± s.e.m.) corresponding to these cases are also shown. Units on axes are μm.
Equation 1 is inapplicable to a non-migratory cell, operationally defined as < 10 μm for t = 100 min. Although a standard statistical technique as described in Refs. 9 and 10 can be used to fit the persistent random walk model to the speed data, model parameters so estimated may not be reliable. For an immotile cell, the displacements between consecutive time points (10 min elapse) were often as small as the position accuracy of our scanning stage system (1 μm), which might produce large errors in the speed data when speeds were low. Therefore, a second parameter, the motile fraction ϕ, was also calculated to distinguish between cells of distinct phenotypes and phenotype changes in the population. The parameter is simply the fraction of cells meeting the operational definition described above.
In addition to centroid positions, ‘stacked’ images were created with either DIAS (Dynamic Image Analysis System, Solltech Inc., Oakdale, IA) or Adobe PhotoShop (San Jose, CA) to allow for appreciation of both positional and morphological changes in a migrating cell. A stacked image consists of filled tracings of the planary projection of the cell at each time point collected overlaid in succession. Both software packages produce comparable cell projection outlines.
Statistical Analysis
Statistical analysis was performed with generalized nonlinear mixed-effect modeling, which employs both nonlinear curve fitting and analysis of variance (ANOVA) to assess the statistical significance of differences in the response variable (i.e. 〈d2〉) and in the fitted model parameters (i.e. μ and P) among the conditions. The latter results were used to infer the effects of (or the lack thereof) the conditions imposed in each experiment. The analysis was carried out using the procedure MIXNLIN (MIXed-effects NonLINear Regression) written for use with the SAS® program (SAS Institute, Cary, N.C.)29.
In some cases, the motile fractions were too low (ϕ < 0.5) for the statistical analysis to be performed, as low displacements caused the matrices in the MIXNLIN procedure to become ill-conditioned. However, these were cases where the motile fractions were so clearly lower than other cases that comparison between motility coefficients was no longer needed.
Cell Adhesion Assay
The adhesion abilities of the Calu-1 and L132 cells to various substrates were also measured. The test cells were labeled with the radioactive 51Cr by adding 350 mCi of Na2CrO4 to the culture medium in a confluent T25 flask and incubating overnight. The labeled cells were detached from the flask with 0.25% Trypsin/5 mM EDTA in PBS and rinsed three times in DMEM with 1% BSA. Then, 3 × 104 of 51Cr-labeled cells were suspended in the appropriate media, added to each well of a 24 or 48 well plate that had been prepared with various matrix proteins or endothelial cell monolayer, and incubated at 37°C for 2 hours. After incubation, the non-adherent cells were removed by gently washing the wells three times with plain DMEM. The adherent cells were lysed with 1% SDS and removed to scintillation tubes with cotton swabs. The radioactivity of each sample collected was measured in a GammaTrac 1191 gamma counter, (Tm Analytic, Elk Grove Village, IL), and compared to standards comprised of reserved cell suspensions identical to the ones incubated in the wells. The assays were performed in triplicate.
Results
A major goal of the present work was to dissect endothelial cell factors responsible for the upregulation of tumor cell motility. The human lung epidermoid carcinoma cell line Calu-1 and its non-malignant counterpart, human embryonic lung epithelial cell line L132 were chosen because they were found in our previous studies to exhibit differential ability to migrate through endothelial cell and reconstituted basement membrane barriers19. In the present work, we compared the relative importance of different categories of possible factors to allow further efforts to be focused on the source(s) which had the most significant effect. Specifically, we intended to determine: 1. Whether the soluble or immobilized endothelial factors had a greater effect; 2. If soluble factors did, whether they were constitutively expressed or induced by tumor cells; 3. If immobilized factors did, which specific ECM protein(s) were responsible.
Effect of Substrate-Bound Endothelial Cell Factors
First, to test if the endothelial cells present surface and ECM molecules that up-regulate the motility of Calu-1 but not L132 cells, the migratory behaviors of both cell lines on live HUVEC monolayers as well as on HUVEC ECM were compared to those of the cells migrating on uncoated tissue culture plastic, hereafter referred to as ‘baseline motility’. Because of their long duration, experiments were performed in an environmental chamber maintained at 37°C and 5% CO2 as in cell culture. Normally epithelial cells were cultured in DMEM supplemented with only FBS (hereafter referred to as DMEM+) whereas HUVECs were cultured in EBM-2 supplemented with growth factors (0.4% hFGF, 0.1% VEGF, and 0.1% hEGF) in addition to FBS (hereafter referred to as EBM-2+). To dissect the contributions of soluble factors to the differential motilities of Calu-1 and L132 cells we had to determine whether their motilities were different in growth factor-enriched EBM-2+ versus growth factor-lacking DMEM+.
As measured by the motility parameters μ (Figure 2A) and ϕ (Figure 2B), on uncoated tissue culture plastic the Calu-1 cells had very low motility in DMEM+, but were motile in the EBM-2+ (which contains more growth factors than the DMEM+). The non-malignant L132 cells did not adhere to the uncoated plastic wells enough to have measurable motility in the DMEM+. In the EBM-2+, however, not only did they adhere to the uncoated plastic, but they were also more motile than the Calu-1 cells (p = 0.0001 for μ). These data suggest an up-regulatory role of the growth factors in EBM-2+.
Figure 2.
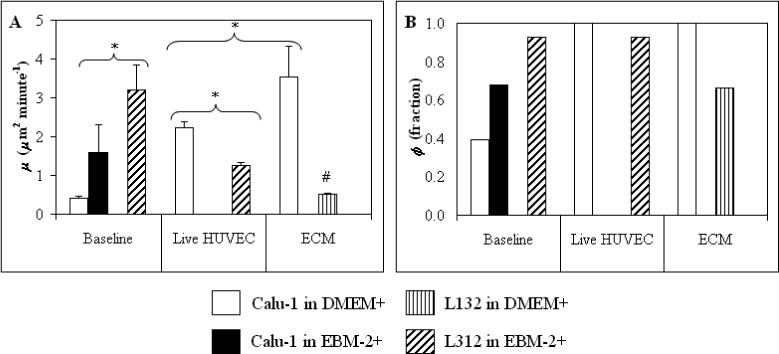
Effects of Insoluble HUVEC Factors on Motility. Motility parameters of indicated cells in the presence and absence of surface factors on HUVEC monolayers and their ECM. * = statistically significant difference between the two experimental conditions indicated by a bracket. # = statistically significant difference from baseline (on uncoated plastic in DMEM+). Error bars = s.e.m.
On the HUVEC monolayer, even though the Calu-1 cells were tested in the DMEM+ and the L132 cells were tested in the EBM-2+, the Calu-1 cells were more motile (double the μ value, p = 0.0003) than the L132 cells. The motile fractions of both cell types were high, indicating that differences seen in the motility parameters reflect differences in the level of migration, instead of a distinction between migratory and non-migratory cells. The Calu-1 cells exhibited an increase in motility, with μ several times the baseline values (no statistics available due to the low baseline motility) and ϕ more than double the baseline value, but the L132 cells exhibited a decrease in motility. The μ value for L132 cells was reduced to less than half of baseline (p = 0.0001), with no change in ϕ.
Additionally, observation of the time-lapse movies revealed that, as a result of the interplay between the Calu-1 cells and the HUVECs, the HUVEC monolayer became partially disrupted, leaving some ECM exposed. Therefore, in the analysis of the Calu-1 migration on a HUVEC monolayer, there were instances where the tumor cells were in fact migrating between the HUVECs on the ECM, rather than exhibiting horizontal locomotion only on the HUVECs themselves.
On the HUVEC ECM, both cell lines were tested in the DMEM+, as they both adhered well. In this case, the Calu-1 cells exhibited high motility, with μ greater on the HUVEC ECM than on a HUVEC monolayer (p = 0.0001). Although nearly 70% of the L132 cells were motile, there was little dispersion, as reflected by the low values of μ.
Therefore, although both cell lines responded to HUVEC factors, the motility of Calu-1 cells was increased while that of the L132 cells was decreased, compared to their respective baseline motility (on uncoated plastic). Again, the similarity in motile fraction indicates that these are differences in the way the cells migrate. In addition, the ECM secreted by the HUVECs had a greater up-regulatory effect on the Calu-1 cell motility than did the HUVECs themselves. This data suggests a major role for the ECM in inducing the motile activities of the tumor cells necessary for successful invasion in the metastasis process.
Lack of Effect of Soluble Factors on Motility
In addition to surface and ECM proteins, the endothelial cells may also secrete soluble molecules to the surrounding environment, both constitutively and in response to the presence of the tumor cells. To dissect effects of such soluble factors, we compared the motile responses of the Calu-1 and L132 cells to soluble factors secreted constitutively by the endothelial cells and secreted in short- (<6 h) or long-term (24 h) response to the presence of the epithelial cells. This was done by measuring the Calu-1 or L132 cell motility on uncoated plastic in EBM-2+ conditioned by the HUVECs for 24 h, with a monolayer of HUVECs on a filter above them to allow for contact between the two cell types via the media only, and in EBM-2+ conditioned 24 h using this co-culture system of the test cells and the endothelial cells, respectively. In addition, to test the effect of soluble factors secreted by the tumor cells themselves, both cell lines were tested in EBM-2+ which had been conditioned by the Calu-1 cells. The results are shown in Figure 3.
Figure 3.
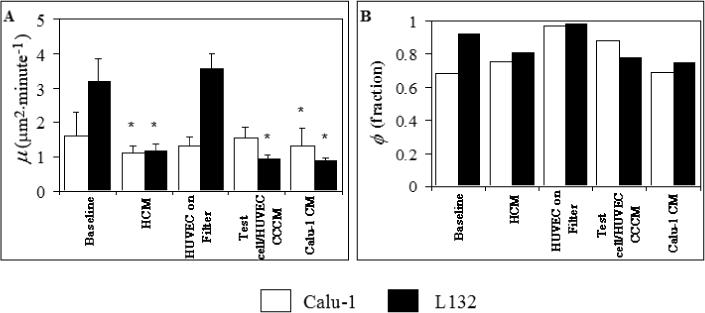
Lack of Effect of Soluble HUVEC Factors on Motility. HCM = HUVEC conditioned media, CCCM = media conditioned with a co-culture of the test cells and the endothelial cells, Calu-1 CM = Calu-1 conditioned media. * = statistically different from baseline (on uncoated plastic in DMEM+). Error bars = s.e.m.
The two cell lines exhibited a motile phenotype in all test conditions, with ϕ values similar to those on plastic in fresh EBM-2+. The μ values of Calu-1 cells were similar in all cases, whereas those of the L132 cells were reduced to less than half of baseline (p < 0.0001) except for the case in which endothelial cells were placed on a filter above the test cells. Thus, although both the Calu-1 and L132 cells had a motile phenotype in the presence of soluble factors secreted by the endothelial cells, their motility coefficients were less then or equal to the corresponding baseline values. Also, the Calu-1 conditioned media data show that Calu-1 soluble factors cannot up-regulate L132 cell motility, although this does not rule out autocrine factors’ affecting Calu-1 motility. The HUVEC conditioned media did, however, have an effect on the morphology of the cells, with the cells displaying significant spreading, greater than that seen in fresh EBM-2+ (see below).
Taken together, these data suggest that there was no upregulation of motility by the soluble factors tested. In some cases, there were decreased levels of motility. Since conditioned media collected after 24 hours of culture were used to test soluble factors, the media used in the motility experiments was likely depleted, at least partly, of nutrients such as growth factors, which may have had a negative effect on cell motility. The L132 cells seemed to be more sensitive to this depletion of nutrients, as their motility was reduced more than was that of the Calu-1 cells in the conditioned media studied.
Morphological Observations
In addition to the motility parameters, differences in the morphology of the migrating cells were also seen between the two cell types and among the various conditions. The cells with low motility usually remained rounded throughout the duration of the experiment, while the cells with high motility generally assumed an elongated shape, typical of a migratory morphology. This result is illustrated in Figure 4, which shows ‘stacked’ representations of the cells in a field of view over an entire six-hour experiment. Panel A shows a case where the Calu-1 cells exhibited a motile phenotype, on fibronectin coated plastic, while panel B shows a case where the Calu-1 cells were not very motile, on vitronectin coated plastic. Note that in addition to the elongation, the migratory cells also spread more than did the non-migratory cells, as indicated by the larger projection areas of the former cells compared to the latter cells. One interesting observation was that of Calu-1 cells migrating on uncoated plastic in media collected from the 24 hour co-culture of tumor cells and endothelial cells (Figure 4C) and in HUVEC conditioned media (not shown). In spite of their low motility in both cases (cf. Figure 3), the Calu-1 cells underwent even more striking morphological changes, developing protrusions in multiple directions, and some immotile Calu-1 cells flattened and spread to cover an unusually large area.
Figure 4.
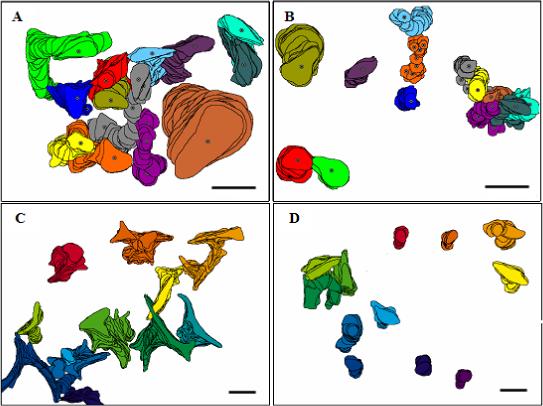
Morphology of Migrating Cells. Planar cell projections at each time point recorded successively were stacked upon each other. Shown are Calu-1 on fibronectin (A), Calu-1 on vitronectin (B), Calu-1 on uncoated plastic in media collected from a tumor cell/endothelial cell 24 hour co-culture (C), and L132 cells on HUVEC ECM (D). Note that cells did not touch each other. Overlaps in the stack images reflect the fact that a cell crossed over the path where another cell had been. Bar = 50 μm. Panels A and B made with DIAS, panels C and D made with Adobe Photoshop.
The L132 cells also developed multiple protrusions in the Calu-1 conditioned media (not shown). The morphology most commonly seen of the L132 cells is illustrated by their stacked images on HUVEC ECM (Figure 4D). Because of their low motility in the majority of cases tested, the L132 cells generally displayed morphology similar to the Calu-1 cells in low migration cases – rounded with little spreading. In cases where the L132 cells were motile, they displayed typical elongated morphology.
Relating Motility on ECM Proteins and Expression of Their Integrin Receptors
Having established that the HUVEC ECM significantly and differentially upregulated the Calu-1 cell motility, we turned to next two questions: 1. which ECM components are responsible and 2. upon which test cell receptors do these ECM proteins act to exert their effects? Two sets of experiments were performed to address these questions. First, the motility of the Calu-1 and L132 cells on isolated ECM proteins collagen I, collagen IV, fibronectin, laminin, and vitronectin, as well as Matrigel and gelatin, was measured. Second, since integrins are known receptors for these proteins, their relative expression on both cell lines was assessed using flow cytometry.
Individual ECM Proteins Up-Regulate Calu-1 Motility
As shown in Figure 5, the Calu-1 motility coefficient μ was increased by collagen I, collagen IV, fibronectin, laminin, and Matrigel, but not by vitronectin or gelatin. In comparison to baseline, the motile fraction, ϕ, of Calu-1 cells was increased by collagen I, collagen IV, fibronectin, and laminin, was unchanged by Matrigel and vitronectin, and was decreased by gelatin. Unlike the baseline case (uncoated plastic), the L132 cells in DMEM+ did adhere enough on all ECM proteins (except for on vitronectin) to allow for measurable motility. However, their motility on collagen I, collagen IV, fibronectin, and laminin was considerably lower than the corresponding Calu-1 motility, with statistically significant differences (p = 0.0001) in μ on all four proteins. In addition, their motile fractions were also low compared to those of the Calu-1 cells in all cases except gelatin. On gelatin the L132 cells exhibited a significantly higher μ than did the Calu-1 cells (p = 0.02).
Figure 5.
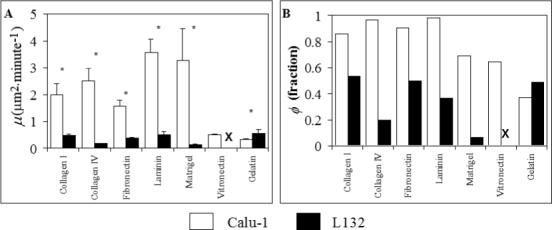
Effects of Isolated ECM Proteins on Motility. All experiments were performed in DMEM+. * = statistically significant difference in μ for Calu-1 and L132 on that protein. Error bars = s.e.m.. ‘X’ indicates adhesion too low for measurable motility. Statistical comparisons of the μ values of Calu-1 cells with their baseline motility (on uncoated plastic in DMEM+) could not be made, as the baseline motility was too low. As the actual binding site density of the different proteins absorbed onto the plastic was not assessed, it is not considered appropriate to statistically compare the motility parameters on the different proteins.
Coating Concentration Affects Motility and Adhesion Behavior
Motility assays were performed on wells coated with 1, 5, 10, 50, and 100 μg mL−1 of laminin to examine the influence of density of substrate-bound proteins, as it has been reported to affect the migratory characteristics of the cells10, 23. The actual amount of absorbed protein was not directly measured in the present work, but studies have shown that in this range of concentrations, the amount of both fibronectin and collagen IV absorbed increases with increasing solution concentration6, 10. As shown in Figure 6, Calu-1 cell migration increased with increasing coating concentration up to a point. Very low motility was seen with coating concentrations of 1 and 5 μg mL−1 – so low that statistical comparisons of the motility with these coating concentrations could not be made. Maximum migration occurred with a coating concentration of 10 μg mL−1. Further increases in coating concentration up to 100 μg mL−1 did not cause significant changes in motility (Figure 6).
Figure 6.
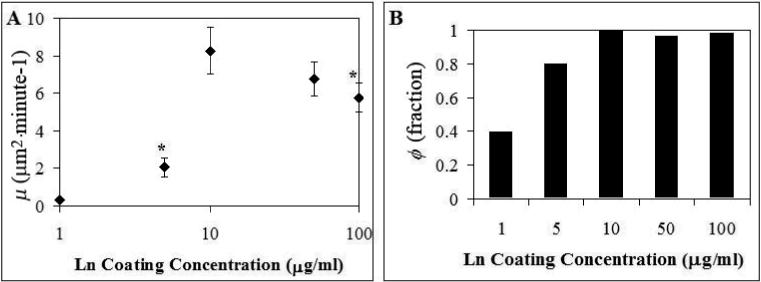
Effect of Varying Laminin Concentration on Motility. The motility parameters calculated from Calu-1 cells migrating on tissue culture plastic coated with solutions containing increasing concentrations of laminin are shown. * indicates statistically significant difference from the point at 10 μg ml−1. Error bars = s.e.m.
Relative Surface Expression of Integrins on Calu-1 and L132 Cells
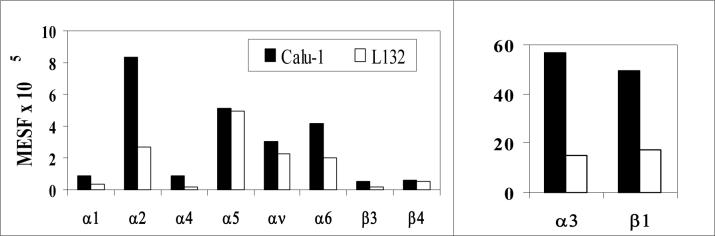
As the above data show, Calu-1, but not L132, cells exhibit upregulated motility on several ECM components tested here, especially laminin and collagen IV. Because integrins are known receptors for ECM proteins, we next determined the surface expression of specific integrins on the two test cells by flow cytometry. Of interest is what, if any, differences there are in the integrin expression levels on the Calu-1 cells and on the L132 cells. It can be seen from Figure 7 that the α2, α3, α6, and β1 integrin subunits had high levels of expression on both cell types, but were expressed at significantly higher levels on the Calu-1 cells than on the L132 cells. High, but comparable levels of α5 and αv integrin subunits were expressed on the two cell types. Very low levels of α1, α4, β3, and β4 integrins were expressed on both cell types.
Figure 7.
Relative Integrin Expression Levels on Calu-1 and L132 Cells. MESF values are presented with the background (negative control – X63) subtracted off. To assure that the results were not biased by subsaturating binding of primary antibodies, mAb concentrations were titrated to saturating levels with serial dilution. The measurements of expression levels of a given integrin subunit on both cell types were obtained in the same experiment.
Published work indicates that the ligands for the α2β1 integrin are collagen and laminin, the ligands for the α3β1 integrin are fibronectin, laminin, and collagen, and the ligand for the α6β1 integrin is laminin12, 20, 28. The results described above show that the Calu-1 cells had significantly higher motility than the L132 cells on each of the ligands of these integrins – collagen I, collagen IV, laminin, and fibronectin. The Calu-1 cells also had higher motility than the L132 cells on Matrigel, which is mostly composed of laminin and collagen IV. This correlation between observed motile behavior and integrin expression levels suggests a possible mechanism for the differences in motility of the two cell types – the Calu-1 cells are able to migrate better on these substrates because they have more receptors for them.
Discussion
Previous studies of tumor cell motility have largely been focused on Boyden Chamber assays to measure tumor cell migration through filters stimulated by soluble factors (chemotaxis)25,27 or by substratum-bound factors (haptotaxis)20,27 and invasion through filters coated with ECM proteins7,14,15,24 or with endothelial cell monolayers grown on them2,19,22. Whereas some studies, such as those employing the radial dish assay5,13, reported direct observation of tumor cell responses to endothelial cell factors, no published work has employed multiple measures of motility to allow for in-depth analysis of migration. Here we used time lapse videomicroscopy and cell track analysis, a method that has become increasingly used in cell motility studies4,6,7,16,18,23,30, to analyze tumor cell motility and compare it with that of non-metastatic cells.
The Calu-1 cell motility was found to be significantly upregulated by monolayers of HUVECs, their ECM, and purified ECM proteins, as compared to both baseline motility and to the corresponding motility of the L132 cells. These findings are in agreement with previous work showing that cells with increased metastatic potential have an increased ability to invade endothelial cell monolayers and reconstituted basement membranes in the transwell assay19, establishing the correlation between the two assays.
Both the malignant and non-malignant cells were found to be migratory in the presence of soluble factors secreted by the endothelial cells, including factors secreted constitutively and in response to the presence of tumor cells, but no increase in motility of either cell line over baseline values was seen. Such soluble factors were not totally without effect upon the cells, as in some cases the cells were seen to spread to a greater degree in HUVEC conditioned media than in normal culture media. Soluble autocrine motility factors, after having been separated from tumor cell supernatant and concentrated, have been reported to upregulate tumor cell motility20,26,27. However, our data show no motility upregulation by media conditioned by the tumor cells, possibly because at the concentrations secreted by the cells, ECM plays a more significant role in motility. Alternately, it is possible that depletion of nutrients such as growth factors during the media conditioning period affected motility in such a way as to mask the effects of autocrine factors.
To further investigate the effect of HUVEC ECM on the Calu-1 cell motility, the cells’ responses to individual proteins commonly found in ECM were measured. The Calu-1 cell motility was found to be significantly upregulated by collagen I, collagen IV, fibronectin, laminin, and Matrigel (but not by vitronectin and gelatin) to levels much higher than that of L132 cells, which showed little motile response to these ECM proteins.
Because adhesion is a critical element of motility, the relative adhesion levels of the two cell types under many of the conditions tested for motility were also measured and examined for possible correlation between adhesion and motility. Initial adhesion levels (assessed after 30 min of incubation) are plotted against motility of the Calu-1 and L132 cells (as measured by μ) in Figure 8. For the Calu-1 cells (Figure 8A), three high motility data points correspond to those with intermediate initial adhesion levels (ECM, laminin, and Matrigel), three low motility data points correspond to low-intermediate adhesions (vitronectin, plastic, and gelatin), and four intermediate motility data points correspond to very low to intermediate-high adhesions (HUVEC monolayers, HUVEC media, collagen I, collagen IV, and fibronectin). This is different from the biphasic relationship between adhesion and motility observed in a previous report23. Similarly, a biphasic relationship is not apparent for the L132 cells (Figure 8B), which lack expression of several integrins. This indicates that, although adhesion is critical for motility, the relationship between the two likely depends on other functional aspects of the adhesive receptors, such as signaling.
Figure 8.
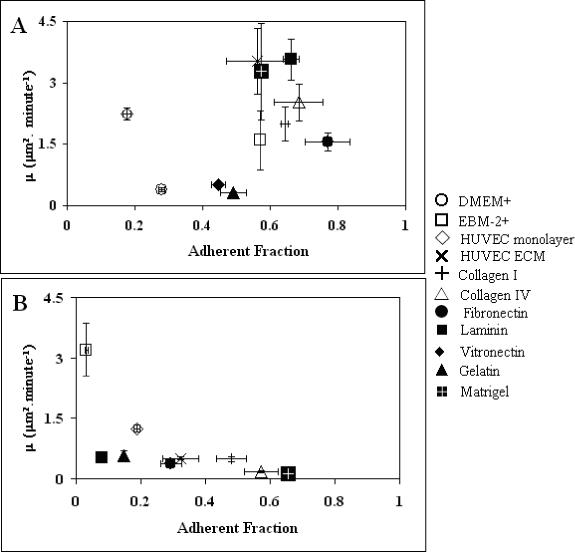
(A) Calu-1 and (B) L132 Motility vs. Adhesion. Motility, as measured by μ, of Calu-1 or L132 cells on various substrates versus adhesion fraction with a thirty minute incubation. For Calu-1 cells, highest motility correlates with intermediate adhesion, while low motility correlated with either high or low adhesion, with the possible exception of migration on a HUVEC monolayer. For L132 cells, although a wide range of initial adhesion levels were seen, motility was uniformly low.
The results of the present study corroborate published work on integrin/ECM protein interactions as related to cell motility, and indicate integrins as the likely molecules used by the Calu-1 cells to upregulate motility in response to HUVEC factors. Of these, the α2β1, α3β1, and α6β1 integrins are prime candidates because the expression of their subunits were significantly higher on the Calu-1 cells than on the L132 cells, which correlates with the differential motility of the two cell types on collagen I and IV, laminin, and fibronectin, known ligands for these integrins. α1, α4, β3, and β4 integrins are unlikely candidates because of their very low expressions on both cell types. Note that, except for Matrigel, the coating concentration of all proteins was 10 μg mL−1, which is the optimal for Calu-1 cells to migrate on laminin (Figure 6). This may not be the optimal coating concentration for L132 cells. It is possible that the reason for the observed differential mobility between the two cells is that the Calu-1 cells were migrating on the ECM at a near optimal density of adhesion ligand for their level of appropriate integrin, while the L132 cells are either are not firmly adhered or too strongly adhered for rapid motility. Further dissection of the molecular mechanisms for regulation of the Calu-1 motility requires identifying the functional roles of these integrins such as by using blocking antibodies in the motility assay, which is the subject of future studies.
Acknowledgments
We thank Dr. D. Soll and the W.M. Keck Dynamic Image Analysis Facility for providing financial support, access to, and training for AW to use the DIAS software for morphological analysis. This work was supported by NSF grant BCS9350370 and NIH grant AI38282. AW was a recipient of NSF Graduate Fellowship, and she was partially supported by an NIH Predoctoral Traineeship (GM 08433) and a Whitaker Foundation Traineeship.
Footnotes
Publisher's Disclaimer: This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2−3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
References
- 1.Alt W. Biased random walk models for chemotaxis and related diffusion approximations. J. Math. Bio. 1980;9:147–177. doi: 10.1007/BF00275919. [DOI] [PubMed] [Google Scholar]
- 2.Belloni PN, Nicolson GL. Role of the vascular endothelium in cancer metastsis. 1992 Plenum Press; New York, NY: pp. 395–425. [Google Scholar]
- 3.Boxberger HJ, Paweletz N, Spiess E, Kriehuber R. An in vitro model study of BSp73 rat tumour cell invasion into endothelial monolayer. Anticancer Res. 1989;9:1777–1786. [Google Scholar]
- 4.Chettibi S, Lawrence AJ, Young JD, Lawrence PD, Stevenson RD. Dispersive locomotion of human neutrophils in response to a steroid- induced factor from monocytes. J. Cell Sci. 1994;107:3173–3181. doi: 10.1242/jcs.107.11.3173. [DOI] [PubMed] [Google Scholar]
- 5.Chicoine MR, Silbergeld DL. Assessment of brain tumor cell motility in vivo and in vitro. J. Neurosurg. 1995;82:615–622. doi: 10.3171/jns.1995.82.4.0615. [DOI] [PubMed] [Google Scholar]
- 6.Chon JH, Netzel R, Rock BM, Chaikof EL. Alpha 4 beta 1 and alpha 5 beta 1 control cell migration on fibronectin by differentially regulation cell speed and motile cell phenotype. Ann. Biomed. Eng. 1998;26:1091–1101. doi: 10.1114/1.139. [DOI] [PubMed] [Google Scholar]
- 7.Chu YW, Runyan RB, Oshima RG, Hendrix MJ. Expression of complete keratin filaments in mouse L cells augments cell migration and invasion. Proc. Natl. Acad. Sci. U S A. 1993;90:4261–4265. doi: 10.1073/pnas.90.9.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu YW, Seftor EA, Romer LH, Hendrix MJ. Experimental coexpression of vimentin and keratin intermediate filaments in human melanoma cells augments motility. Am. J. Pathol. 1996;148:63–69. [PMC free article] [PubMed] [Google Scholar]
- 9.Dickinson RB, Tranquillo RT. Optimal estimation of cell movement indices from the statistical analysis of cell tracking data. AIChE. J. 1993;39:1995–2010. [Google Scholar]
- 10.DiMilla PA, Stone JA, Quinn JA, Albelda SM, Lauffenburger DA. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J. Cell Biol. 1993;122:729–737. doi: 10.1083/jcb.122.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn GA. Characterizing a kenesis response: time averaged measure of cell speed and directional persistence. Agents Actions Suppl. 1983;12:14–33. doi: 10.1007/978-3-0348-9352-7_1. [DOI] [PubMed] [Google Scholar]
- 12.Elangbam CS, Qualls CW, Jr., Dahlgren RR. Cell adhesion molecules--update. Vet. Pathol. 1997;34:61–73. doi: 10.1177/030098589703400113. [DOI] [PubMed] [Google Scholar]
- 13.Friedlander DR, Zagzag D, Shiff B, Cohen H, Allen JC, Kelly PJ, Grumet M. Migration of brain tumor cells on extracellular matrix proteins in vitro correlates with tumor type and grade and involves alphaV and beta1 integrins. Cancer Res. 1996;56:1939–1947. [PubMed] [Google Scholar]
- 14.Garden RJ, Liu BC, Redwood SM, Weiss RE, Droller MJ. Bacillus Calmette-Guerin abrogates in vitro invasion and motility of human bladder tumor cells via fibronectin interaction. J. Urol. 1992;148:900–905. doi: 10.1016/s0022-5347(17)36774-5. [DOI] [PubMed] [Google Scholar]
- 15.Ho WC, Heinemann C, Hangan D, Uniyal S, Morris VL, Chan BM. Modulation of in vivo migratory function of alpha 2 beta 1 integrin in mouse liver. Mol. Biol. Cell. 1997;8:1863–1875. doi: 10.1091/mbc.8.10.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huttenlocher A, Ginsberg MH, Horwitz AF. Modulation of cell migration by integrin-mediated cytoskeletal linkages and ligand-binding affinity. J. Cell Biol. 1996;134:1551–1562. doi: 10.1083/jcb.134.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer RH, Nicolson GL. Interactions of tumor cells with vascular endothelial cell monolayers: a model for metastatic invasion. Proc. Natl. Acad. Sci. U S A. 1979;76:5704–5708. doi: 10.1073/pnas.76.11.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee Y, McIntire LV, Zygourakis K. Analysis of endothelial cell locomotion: differential effects of motility and contact inhibition. Biotechnol. Bioeng. 1994;43:622–634. doi: 10.1002/bit.260430712. [DOI] [PubMed] [Google Scholar]
- 19.Li Y-H, Zhu C. A modified boyden chamber assay for tumor cell transendothelial migration in vitro. Clin. Exp. Metastasis. 1999;17:423–429. doi: 10.1023/a:1006614232388. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy JB, Skubitz AP, Iida J, Mooradian DL, Wilke MS, Furcht LT. Tumor cell adhesive mechanisms and their relationship to metastasis. Semin. Cancer Biol. 1991;2:155–167. [PubMed] [Google Scholar]
- 21.Ohigashi H, Shinkai K, Mukai M, Ishikawa O, Imaoka S, Iwanaga T, Akedo H. In vitro invasion of endothelial cell monolayer by rat ascites hepatoma cells. Jpn. J. Cancer Res. 1989;80:818–821. doi: 10.1111/j.1349-7006.1989.tb01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okada T, Okuno H, Mitsui Y. A novel in vitro assay system for transendothelial tumor cell invasion: significance of E-selectin and α3 integrin in the transendothelial invasion by HT1080 fibrosarcoma cells. Clin. Exp. Metastasis. 1994;12:305–314. doi: 10.1007/BF01753837. [DOI] [PubMed] [Google Scholar]
- 23.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 24.Repesh LA, Drake SR, Warner MC, Downing SW, Jyring R, Seftor EA, Hendrix MJ, McCarthy JB. Adriamycin-induced inhibition of melanoma cell invasion is correlated with decreases in tumor cell motility and increases in focal contact formation. Clin. Exp. Metastasis. 1993;11:91–102. doi: 10.1007/BF00880070. [DOI] [PubMed] [Google Scholar]
- 25.Savarese DM, Russell JT, Fatatis A, Liotta LA. Type IV collagen stimulates an increase in intracellular calcium. Potential role in tumor cell motility. J. Biol. Chem. 1992;267:21928–21935. [PubMed] [Google Scholar]
- 26.Silletti SA. Autocrine motility factor and its receptor: on the role of tumor cell motility in metastasis (Abs only). Diss. Abstr. Int. [B] 1995;56:2424. [Google Scholar]
- 27.Stracke M, Liotta LA, Schiffmann E. The role of autotaxin and other motility stimulating factors in the regulation of tumor cell motility. Symp. Soc. Exp. Biol. 1993;47:197–214. [PubMed] [Google Scholar]
- 28.Tryggvason K. The laminin family. Curr. Opin. Cell Biol. 1993;5:877–82. doi: 10.1016/0955-0674(93)90038-r. [DOI] [PubMed] [Google Scholar]
- 29.Vonesh EF, Chinchilli VM. Linear and nonlinear models for the analysis of repeated measurements. Marcel Dekker, Inc.; New York: 1997. p. 560. [Google Scholar]
- 30.Ware MF, Wells A, Lauffenburger DA. Epidermal growth factor alters fibroblast migration speed and directional persistence reciprocally and in a matrix-dependent manner. J. Cell Sci. 1998;111:2423–2432. doi: 10.1242/jcs.111.16.2423. [DOI] [PubMed] [Google Scholar]
- 31.Wright A. M.S. thesis. School of Mechanical Engineering, Georgia Institute of Technology; Atlanta, GA.: 1997. Design, Development, and Application of an Automated Precision Scanning Microscope State with a Controlled Environment. [Google Scholar]