Abstract
Capillary electrophoresis mass spectrometry (CE/MS) was applied for the comprehensive survey of changes in the amounts of metabolites upon the shift from photoautotrophic to photomixotrophic conditions in Synechocystis sp. PCC 6803. When glucose was added to the photoautotrophically grown culture, the increase in the metabolites for the oxidative pentose phosphate (OPP) pathway and glycolysis, together with the decrease in those for the Calvin cycle, was observed. Concomitantly, the increase in respiratory activity and the decrease in photosynthetic activity took place in the wild-type cells. In the pmgA-disrupted mutant that shows growth inhibition under photomixotrophic conditions, lower enzymatic activities of the OPP pathway and higher photosynthetic activity were observed, irrespective of trophic conditions. These defects brought about metabolic disorders such as a decrease in ATP and NADPH contents, a failure in the activation of respiratory activity, and the aberrant accumulation of isocitrate under photomixotrophic but not under photoautotrophic conditions. A delicate balancing of the carbon flow between the Calvin cycle and the OPP pathway seems indispensable for growth specifically under photomixotrophic conditions and PmgA is likely to be involved in the regulation.
Keywords: CE/MS, cyanobacteria, glucose, metabolome, photomixotrophy, pmgA
Introduction
Photosynthetic organisms face continuous constraints in their growth environment. In order to meet the energy demands for sustainable life, cyanobacteria show versatile growth characteristics in response to the availability of light and the carbon source (Rippka et al., 1979; Stal and Moezelaar, 1997). They are primarily photoautotrophic organisms performing oxygenic photosynthesis to convert light energy to chemical energy. ATP and NADPH generated by photosynthesis are used for the assimilation of CO2 by the Calvin cycle and the excess carbon fixed is stored in the form of glycogen. Some cyanobacterial species have the ability of heterotrophic energy generation in addition to the photosynthetic capability: they catabolize glucose via the oxidative pentose phosphate (OPP) pathway, the glycolytic pathway, and an incomplete tricarboxylic acid (TCA) cycle to produce ATP, NADPH, and carbon skeletons used as anabolic precursors. In cyanobacteria having no organelle, all of the above-mentioned metabolisms, CO2 fixation, gluconeogenesis, and glycolysis, are performed in the cytoplasm and several enzymes are shared among these pathways (Smith, 1982). Thus, the control of functionally equivalent reactions in the anabolic and catabolic pathways seems to be a prerequisite for optimized growth under different trophic conditions.
The alteration in the metabolic processes in response to the environmental change has been examined using a glucose-tolerant strain of Synechocystis sp. PCC 6803 that is capable of photoautotrophic, photomixotrophic, and heterotrophic growth. The availability of light energy significantly affects the expression level of mRNA (Gill et al., 2002), that of proteins (Yang et al., 2002a; Kurian et al., 2006) and cellular metabolism (Yang et al., 2002a) in Synechocystis. When cultures are transferred from photoautotrophic to dark heterotrophic conditions with glucose, CO2 assimilation by the Calvin cycle is rapidly inactivated and the major metabolic pathway switches from the Calvin cycle to the OPP pathway (Pelroy et al., 1972). On the other hand, the addition of glucose in the presence of light, namely, the shift from photoautotrophic to photomixotrophic conditions, hardly affects the expression pattern of transcripts (Yang et al., 2002a; Kahlon et al., 2006) and of proteins (Yang et al., 2002a; Herranen et al., 2004). Under photomixotrophic conditions where both light and glucose are available, substantial activity of the Calvin cycle is detected (Yang et al., 2002a, b, c). Early works showed that the OPP pathway is repressed in the presence of light through the allosteric inhibition of glucose-6-phosphate dehydrogenase (G6PDH) by ribulose-1,5-diphosphate (RuBP), an intermediate of the Calvin cycle (Pelroy et al., 1972). Thus, the metabolic characteristic of photomixotrophic cultures has been considered to be similar to that of photoautotrophic ones: it is largely dependent on photosynthesis but not on glucose catabolism. However, recent studies reported the detection of high G6PDH activity in photomixotrophically grown cells (Knowles and Plaxton, 2003; Singh and Sherman, 2005; Kahlon et al., 2006). This implies that both the Calvin cycle and the OPP pathway, whose enzymatic reactions proceed in the reverse direction for the most part, can coexist under photomixotrophic conditions. If this is the case, there must be a regulatory mechanism to co-ordinate anabolic and catabolic activities upon the shift from photoautotrophic to photomixotrophic conditions. At present, information on such a regulatory mechanism is not available, since there is no comprehensive study focused on the difference in metabolic processes under these two trophic conditions.
In this study, two strategies were used to address the mechanism that enables Synechocystis sp. PCC 6803 to grow photomixotrophically. First, capillary electrophoresis mass spectrometry (CE/MS) was applied for the comprehensive survey of metabolic processes, since this method is useful for the simultaneous detection of metabolites, especially charged ones such as organic acids and nucleotides. In previous reports, the CE/MS method successfully revealed metabolite alterations in Bacillus subtilis (Soga et al., 2002), Oryza sativa (Sato et al., 2004; Takahashi et al., 2006a) and Arabidopsis thaliana (Takahashi et al., 2006b). Second, to unravel the regulatory mechanism required for photomixotrophic growth, the amounts of metabolites were compared between the wild-type and a mutant showing sensitivity to photomixotrophic conditions. The gene-disrupted mutant of pmgA (sll1968) encoding a putative regulatory protein can grow normally under photoautotrophic or heterotrophic conditions, but suffers severe growth inhibition under photomixotrophic conditions (Hihara and Ikeuchi, 1997). Metabolic characterization of such a regulatory mutant showing light/glucose sensitivity seems a good approach to clarify the regulatory processes required for photomixotrophic growth.
Materials and methods
Strains and culture conditions
A glucose-tolerant wild-type strain of Synechocystis sp. PCC 6803 and the pmgA-disrupted mutant made by inserting the spectinomycin resistance cassette (Hihara and Ikeuchi, 1997) were used for the study. They were grown at 32 °C in BG-11 medium with 20 mM HEPES-NaOH, pH 7.0, under continuous illumination of 50 μmol photons m−2 s−1. Cultures were grown in volumes of 50 ml in test tubes (3 cm in diameter) and bubbled with air supplemented with 1% CO2. Cell density was estimated at A730 using a spectrophotometer (model UV-160A, Shimadzu, Kyoto, Japan). For the comparison of a photomixotrophic culture with a photoautotrophic one, photoautotrophically grown cultures at mid-log phase were inoculated into the fresh medium with or without 5 mM glucose at A730=0.05–0.1 and incubated at 50 μmol photons m−2 s−1 for the indicated duration.
Metabolite analysis
Quantification of metabolites was performed using the method described by Takahashi et al. (2006b). Fifty ml of cultures were harvested by centrifugation at 15 000 g at 4 °C for 2 min and the cell pellets obtained (30–50 mg in fresh weight) were frozen in liquid nitrogen. Samples were vortexed with 200 μl of ice-cooled 50% (v/v) methanol containing internal standards (50 μM PIPES) for 10 min. The supernatant was recovered by centrifugation at 15 000 g at 4 °C for 5 min, filtered with a 5 kDa cut-off filter (Millipore, Bedford, MA, USA), and used for analysis by CE/MS. Separation of metabolites was performed on a polyethylene glycol-coated capillary (DB-WAX, 100 cm×50 μm i.d., J&W Scientific, Folsom, CA, USA) using 20 mM ammonium acetate, pH 6.8, as a running buffer. Metabolites in the extract were identified by comparison of the migration time and m/z ratio with those of authentic organic acids and nucleotides. The quantification was performed by comparing peak areas of metabolites in samples with those of the authentic standards. As for glyceraldehyde-3-phosphate (GA3P) that exists as both the labile diol and the stable aldehyde forms in aqueous solution (Trentham et al., 1969), the stable form was specifically detected by CE/MS.
Measurement of enzymatic activities of cells
Fifty ml of cultures were harvested by brief centrifugation and resuspended with 500 μl of 50 mM sodium phosphate buffer, pH 7.5, containing 3 mM MgCl2 and 1 mM DTT. Approximately 250 μl volume of zircon beads (0.1 mm in diameter, Biospec, Bartlesville, OK, USA) were added to the cell suspension and the cells were broken by vigorous vortexing for 2 min followed by cooling on ice for 1 min and this procedure was repeated four times. The lysate was centrifuged at 15 000 g for 10 min and the resulting supernatant was used for the measurement.
NADP+-specific enzymatic activities in cell extracts were measured spectrophotometrically by monitoring the substrate-dependent generation of NADPH at 340 nm. For measurements of the activities of glucose-6-phosphate dehydrogenase (G6PDH), 6-phosphogluconate dehydrogenase (6PGDH), and isocitrate dehydrogenase (ICDH), the reaction mixture containing 100 mM sodium phosphate buffer, pH 7.5, 3 mM MgCl2, 0.4 mM NADP+ and the cell extract in a total volume of 1 ml was used. For measurements of aconitase activity, the reaction mixture containing 100 mM sodium phosphate buffer, pH 7.5, 15 mM MgCl2, 1 mM NADP+, 1 mM EDTA, the cell extract, and NADP+-dependent ICDH (0.6 units) in a total volume of 1 ml was used. Reactions were started by the addition of respective substrates at 30 °C. Five mM glucose-6-phosphate, 5 mM 6-phosphogluconate, 2.5 mM isocitrate, and 30 mM cis-aconitate were added as substrates for the measurements of G6PDH, 6PGDH, ICDH, and aconitase, respectively. 1 U of enzymatic activity corresponds to the formation of 1 μmol NADPH/min under standard assay conditions.
Protein concentration was determined using Protein Assay Kits II (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer's instructions.
Measurement of photosynthetic and respiratory activities of cells
An aliquot (1 ml) of photoautotrophically or photomixotrophically grown cultures was placed in a Clark-type oxygen electrode chamber and stirred gently at 30 °C. Whole-cell photosynthetic activity was measured as oxygen evolution supported by 2 mM NaHCO3 at 50 μmol photons m−2 s−1. Respiratory activity was measured as oxygen consumption in the presence of 5 mM glucose in the dark. The rates of oxygen evolution and consumption were calculated in terms of μmol oxygen evolved (108 cells)−1 h−1 and of μmol oxygen consumed (108 cells)−1 h−1, respectively.
Results
Growth properties of the wild-type and pmgA-disrupted mutant cells under photomixotrophic conditions
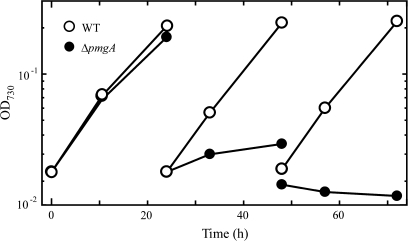
First, the growth properties of the wild-type cells and pmgA-disrupted mutant cells (ΔpmgA mutant) were examined under photomixotrophic conditions (Fig. 1). Five mM glucose was added to the photoautotrophically grown cultures at time 0. When the cultures were diluted every 24 h to minimize the self-shading effect, the delay in the growth of the ΔpmgA mutant became prominent on the second day. The growth of the mutant completely stopped on the third day, whereas the wild-type cells did not suffer any growth inhibition. If the mutant has any defects in the regulation of metabolic processes, aberrant levels of metabolites should be detected preceding the growth inhibition. Thus, the time point of 24 h after the addition of glucose was chosen for the sampling time to examine the amounts of metabolites in cultures incubated under photomixotrophic conditions for 24 h.
Fig. 1.
Growth curve of the wild-type (open circles) and ΔpmgA (closed circles) cells in liquid BG-11 medium supplemented with 5 mM glucose. Cultures grown at 50 μmol photons m−2 s−1 were supplemented with glucose at time 0, and diluted every 24 h to avoid the self shading of cells.
Amounts of metabolites in glycolysis, the OPP pathway, and the Calvin cycle
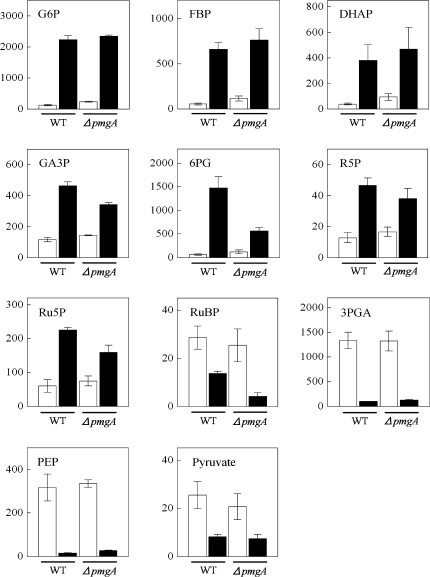
The amounts of metabolites engaged in the central carbon metabolic pathway in cyanobacteria (Fig. 2) were examined using CE/MS. Figure 3 shows the amounts of metabolites in glycolysis, the OPP pathway, and the Calvin cycle in wild-type and ΔpmgA mutant cells incubated under photoautotrophic or photomixotrophic conditions for 24 h. The presence of glucose had a great impact on the amounts of these metabolites in both strains. Namely, the levels of glucose-6-phosphate (G6P), fructose-1,6-bisphosphate (FBP), dihydroxyacetone phosphate (DHAP), glyceraldehyde-3-phosphate (GA3P), 6-phosphogluconate (6PG), ribose-5-phosphate (R5P), and ribulose-5-phosphate (Ru5P) increased significantly, while those of RuBP, 3-phosphoglycerate (3PGA), phosphoenolpyruvate (PEP), and pyruvate showed a marked decrease. It is notable that GA3P, 6PG, Ru5P, and RuBP contents were lower in the mutant than in the wild-type under photomixotrophic conditions.
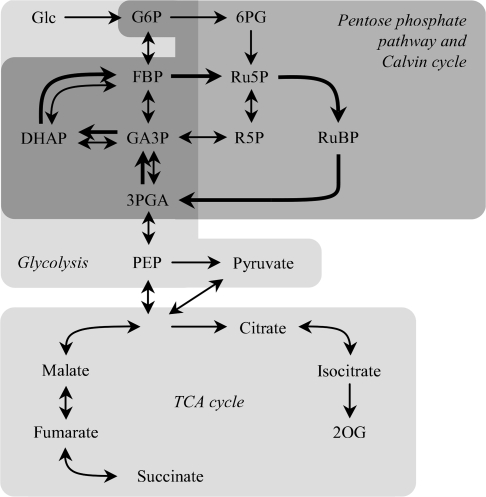
Fig. 2.
The central metabolic pathway of Synechocystis sp. PCC 6803 in the integration of metabolites described in this study. Thick arrows indicate the direction of the metabolic flow of the Calvin cycle.
Fig. 3.
Amounts of metabolites in glycolysis, the OPP pathway and the Calvin cycle. Wild-type and ΔpmgA mutant were grown photoautotrophically (white bars) or photomixotrophically (black bars) for 24 h, and then metabolite contents were quantified. Each value represents the means ±SD of three independent experiments (nmol g−1 fresh weight).
Amounts of metabolites in the TCA cycle
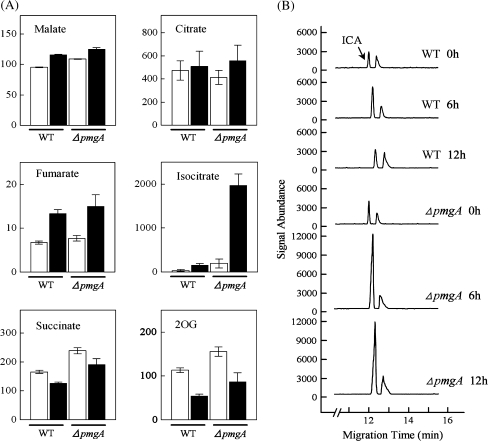
Next, the contents of metabolites related to the TCA cycle were examined (Fig. 4A). Incubation under photomixotrophic conditions led to the increase in contents of malate, fumarate, and isocitrate, while succinate and 2-oxoglutarate (2-OG) contents decreased in both strains. Surprisingly, the isocitrate content in the ΔpmgA mutant increased up to 10-fold on incubation with glucose, whereas that in the wild-type showed only a slight increase. Then the time-course of isocitrate accumulation was examined after the addition of glucose (Fig. 4B). In the wild-type cells, isocitrate content was slightly increased, while it showed an approximately 10-fold increase within 6 h in the mutant and stayed at an almost constant level until 24 h after glucose addition.
Fig. 4.
Amounts of metabolites in the TCA cycle. (A) Comparison of metabolite contents in wild-type and the ΔpmgA mutant cells grown photoautotrophically (white bars) or photomixotrophically (black bars) for 24 h. Each value represents the means ±SD of three independent experiments (nmol g−1 fresh weight). (B) The time-course of isocitrate (ICA) accumulation after the addition of glucose. Electropherograms (m/z 191) were obtained every 6 h after glucose addition using CE/MS and signal abundances were normalized to fresh weight and internal standard level.
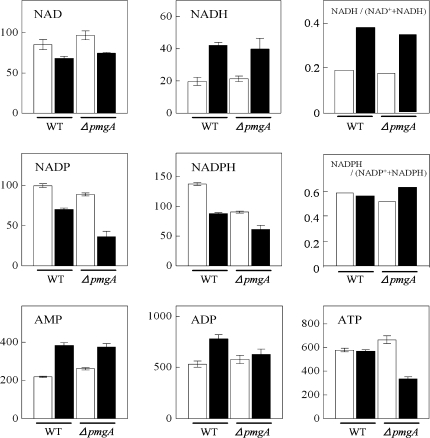
Amounts of adenine and pyridine nucleotides
The amounts of adenine and pyridine nucleotides were examined (Fig. 5). In wild-type cells incubated with glucose for 24 h, the increase of NADH, AMP, and ADP contents and the decrease of NAD+, NADP+, and NADPH contents were observed. In the case of ΔpmgA mutant cells under photoautotrophic conditions, the nucleotide contents were almost the same as those in the wild-type cells except for the lower amount of NADPH. On the other hand, under photomixotrophic conditions, NADP+, NADPH, and ATP contents were significantly lower in the mutant. In both strains, the ratio of NADH to total (NAD+ +NADH) increased, whereas that of NADPH to total (NADP+ +NADPH) hardly changed upon the shift to photomixotrophic conditions.
Fig. 5.
Amounts of adenine- and pyridine-nucleotides. Wild-type and ΔpmgA mutants were grown photoautotrophically (white bars) or photomixotrophically (black bars) for 24 h, and then metabolite contents were quantified. Each value represents the means ±SD of three independent experiments (nmol g−1 fresh weight).
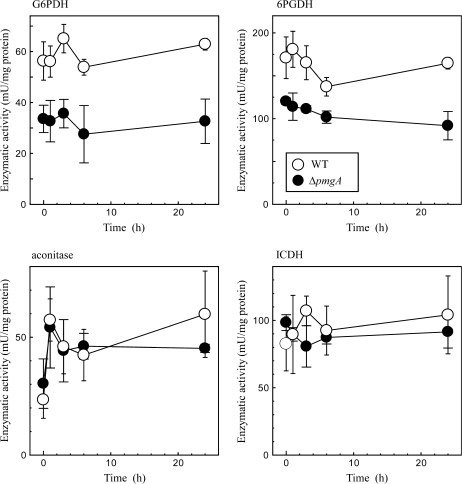
Enzymatic activities
For a better understanding of the changes in metabolite levels, the glucose-dependent changes in enzymatic activities were examined concerning several enzymes that are suspected to show different activities between the wild-type and delta-pmgA mutant cells. Cell extracts were prepared from cultures incubated under photomixotrophic conditions for 0, 1, 3, 6, and 24 h. The activities of G6PDH and 6PGDH that control the carbon flow into the OPP pathway were determined first. As shown in Fig. 6, activities of these enzymes were only slightly affected by the addition of glucose. A notable finding is that these activities in the mutant were considerably lower than those in the wild-type cells, irrespective of trophic conditions.
Fig. 6.
Change in activities of G6PDH, 6PGDH, aconitase, and ICDH in wild-type (open circles) and ΔpmgA (closed circles) cells upon the shift from photoautotrophic to photomixotrophic conditions. Cultures grown at 50 μmol photons m−2 s−1 were supplemented with 5 mM glucose at time 0. Samples were taken at the indicated time points, and enzymatic activities in cell extracts were determined as described in the Materials and methods. Data are the means ±SD of three independent experiments.
The aberrant accumulation of isocitrate in the mutant may be caused by the defect in the isocitrate formation catalysed by aconitase and/or the degradation catalysed by NADP+-dependent isocitrate dehydrogenase (ICDH). Thus, the time-course change of the activity of these enzymes was examined (Fig. 6). The aconitase activity was up-regulated within 1 h, whereas the ICDH activity was hardly affected by the addition of glucose. In both cases, no difference in the enzymatic activity was observed between the wild-type and ΔpmgA mutant. ICDH activity is dependent on the level of NADP+ which was shown to be low in the mutant under photomixotrophic conditions (Fig. 5). Thus, there is a possibility that in vivo activity of ICDH in the mutant is restricted by the availability of NADP+, although such a limitation cannot be observed in the reaction mixture containing sufficient NADP+. To test this possibility, the intracellular level of NADP+ was examined 6 h after the addition of glucose when the accumulation of isocitrate became prominent in the mutant cells (Fig. 4B). At this time point, NADP+ decreased to half of its initial level and there was no difference in the amount between the two strains (not shown). This indicates that the activity of NADP+-ICDH in the mutant is also normally regulated in vivo.
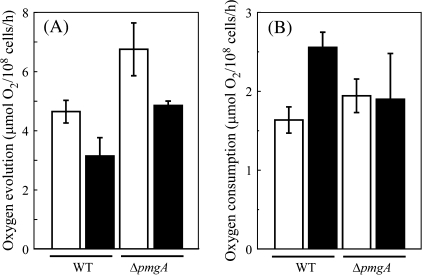
Photosynthetic and respiratory activities
The photosynthetic and respiratory activities of the wild-type and ΔpmgA mutant cells incubated under photoautotrophic or photomixotrophic conditions for 24 h were examined. Photosynthetic activity was measured as the rate of NaHCO3-dependent oxygen evolution under the growth conditions, that is, at an illumination of 50 μmol photons m−2 s−1 with or without 5 mM glucose (Fig. 7A). In the wild-type cells, the oxygen evolution rate decreased to two-thirds of the initial level upon the addition of glucose. The oxygen evolution rate of the ΔpmgA mutant was higher than that of the wild-type under photoautotrophic conditions, which is consistent with the previous observation (Hihara et al., 1998). Although the oxygen evolution rate also decreased in the mutant after the addition of glucose, the resultant activity was still considerably higher than that in the wild type. Respiratory activity was measured as the rate of oxygen consumption under dark conditions in the presence of 5 mM glucose (Fig. 7B). When incubated with glucose for 24 h, the oxygen consumption rate increased 1.5-fold in the wild-type cells, whereas it hardly changed in the ΔpmgA mutant.
Fig. 7.
Photosynthetic (A) and respiratory (B) activities of the wild-type and ΔpmgA cells incubated under photoautotrophic or photomixotrophic conditions for 24 h. Photosynthetic activity was measured as oxygen evolution supported by 2 mM NaHCO3 at 50 μmol photons m−2 s−1. Respiratory activity was measured as oxygen consumption in the presence of 5 mM glucose in the dark. Data are the means ±SD of three independent experiments.
Discussion
The difference in metabolic processes between photoautotrophically and photomixotrophically grown cultures of Synechocystis sp. PCC 6803
In this study, the level of metabolites in Synechocystis cells was significantly affected by the transfer from photoautotrophic to photomixotrophic conditions, irrespective of the almost unchanged levels of transcripts (Yang et al., 2002a; Kahlon et al., 2006) and of proteins (Yang et al., 2002a; Herranen et al., 2004). It is evident that the metabolic balance is shifted toward catabolism by the addition of glucose, although the anabolic pathways are still active unlike in the case of the transfer to heterotrophic conditions.
After 24 h of incubation under photomixotrophic conditions, the amounts of G6P, 6PG, Ru5P, R5P, FBP, GA3P, and DHAP increased (Fig. 3), indicating an increase in carbon flow to the OPP pathway and glycolysis. In cyanobacteria, the major route of glucose catabolism was suggested to be the OPP pathway and the lower part of the glycolytic pathway (Pelroy et al., 1972), whereas the contribution of the upper part of the glycolytic pathway has been elusive. However, our results clearly show that both the OPP pathway and the whole of glycolysis actively participate in glucose catabolism. The increase in NADH content under photomixotrophic conditions (Fig. 5) also indicates the enhanced flow through glycolysis. Measurement of G6PDH and 6PGDH activities revealed that the enzymatic activities themselves are not up-regulated under photomixotrophic conditions (Fig. 6), which is consistent with previous reports (Knowles and Plaxton, 2003; Singh and Sherman, 2005; Kahlon et al., 2006). It is probable that the abundant supply of substrates under photomixotrophic conditions leads to the enhancement of sugar catabolism. Judging from the 1.5-fold higher oxygen consumption rate in photomixotrophically grown cells than that in photoautotrophically grown cells (Fig. 7B), respiratory electron transport seems also to be activated under photomixotrophic conditions.
On the other hand, carbon flow through the Calvin cycle seems to decrease under photomixotrophic conditions, judging from the decrease in the Calvin cycle intermediates (Fig. 3) and photosynthetic activity (Fig. 7A). This indicates that the restriction of photosynthesis is one of the strategies of Synechocystis sp. PCC 6803 to adapt to photomixotrophic condition. The amounts of RuBP, 3PGA, PEP, and pyruvate significantly decreased upon the addition of glucose in contrast to the increase of Ru5P and GA3P (Fig. 3). Phosphoribulokinase (PRK) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) catalysing the conversions of Ru5P and GA3P to RuBP and 3PGA, respectively, are known to be key enzymes for the regulation of carbon flow between the Calvin cycle and the OPP pathway (Wedel and Soll, 1998). The increase in substrates and the decrease in products of these enzymes under photomixotrophic conditions are probably caused by the repression of the activities of PRK and GAPDH. In Synechococcus sp. PCC 7942 cultured under a 12/12 h light/dark cycle, the down-regulation of the Calvin cycle during the dark period was shown to be attained through the complex formation of GAPDH, PRK, and a small protein named CP12 in response to the decrease in the NADP(H)/NAD(H) ratio (Tamoi et al., 2005). It is reasonable to assume that this inhibitory mechanism works in photomixotrophically grown Synechocystis, because a significant decrease was observed in the NADP(H)/NAD(H) ratio on the addition of glucose, from 2.3 to 1.4 in the wild-type cells. Upon the shift to photomixotrophic conditions, the extent of the decrease in the amount of the Calvin cycle intermediates such as RuBP and 3PGA (Fig. 3) was much larger than that in photosynthetic activity (Fig. 7A). It is possible that these metabolites hardly accumulate under photomixotrophic conditions probably due to the increased metabolic flux to the OPP pathway and to the TCA cycle.
Among the metabolites in the TCA cycle, the amount of malate, fumarate, and isocitrate increased under photomixotrophic conditions, whereas that of succinate and 2-OG decreased (Fig. 4A). In cyanobacteria lacking both 2-oxoglutarate dehydrogenase and succinyl-CoA synthetase activities (Pearce et al., 1969), succinate and 2-OG are terminal metabolites of the TCA cycle and serve as a precursor of various biosynthetic reactions. Succinate is a substrate for succinate dehydrogenase (SDH) that is a major component of the cyanobacterial respiratory electron transport chain (Cooley and Vermaas, 2001). On the other hand, 2-OG serves as a carbon skeleton required for nitrogen assimilation and also works as a regulatory metabolite involved in the co-ordination between carbon and nitrogen metabolism (Muro-Pastor et al., 2001). Enhancemenet of SDH activity and nitrogen assimilation may be the cause of the decrease in these metabolites under photomixotrophic conditions.
The difference in metabolic processes between the wild-type and ΔpmgA mutant cells under photomixotrophic conditions
Growth of the ΔpmgA mutant is severely inhibited after 24 h of incubation under photomixotrophic conditions, although the wild-type cells can grow normally. CE/MS analysis and measurement of enzymatic, photosynthetic, and respiratory activities suggested that the ΔpmgA mutant has a defect in the co-ordination of anabolic and catabolic activities, leading to a metabolic imbalance and growth inhibition under photomixotrophic conditions.
The low amount of 6PG, Ru5P, R5P (Fig. 3), and NADPH (Fig. 5) together with the low activities of G6PDH and 6PGDH (Fig. 6) observed in the photomixotrophically grown ΔpmgA mutant indicated the decreased carbon flow to the OPP pathway. The respiratory activity of ΔpmgA mutant cells was not up-regulated under photomixotrophic conditions (Fig. 7B), which is probably due to the smaller amount of NADPH. Furthermore, the low respiratory activity in the mutant may result in a decreased rate of ATP synthesis through oxidative phosphorylation (Fig. 5).
The decrease in photosynthetic activity upon the shift to photomixotrophic conditions was observed in the ΔpmgA mutant as well as in the wild type (Fig. 7A). The amounts of RuBP, 3PGA, PEP, and pyruvate largely decreased in the both strains (Fig. 3), suggesting that the inhibitory mechanism of PRK and GAPDH normally operates in the mutant. However, the ΔpmgA mutant still showed a higher rate of photosynthetic activity than the wild type under photomixotrophic conditions (Fig. 7A). Active CO2 fixation, together with the slowdown in the regeneration of RuBP, the substrate of CO2 fixation, is likely to cause the decrease in the RuBP level in the mutant (Fig. 3).
The most notable difference between the wild type and the ΔpmgA mutant was seen in the isocitrate content under photomixotrophic conditions. Aberrant accumulation of isocitrate was observed in ΔpmgA mutant cells within 6 h after the addition of glucose (Fig. 4B). The conversion of citrate to isocitrate catalysed by aconitase and that of isocitrate to 2-OG catalysed by NADP+-ICDH were normally operated in the ΔpmgA mutant (Fig. 6). Perhaps the higher activity of the Calvin cycle in the ΔpmgA mutant brings about the excess supply of carbon materials, leading to the isocitrate accumulation. Sakuragi et al. (2006) reported that the ΔpmgA mutant accumulated twice as much total sugar as the wild type under photoautotrophic and photomixotrophic conditions. This observation also indicates an excess carbon flow within ΔpmgA mutant cells.
It is of note that low enzymatic activities in the OPP pathway (Fig. 6) and high photosynthetic activity (Fig. 7A) can be observed in the mutant not only under photomixotrophic but also under photoautotrophic conditions. In spite of these defects, the amounts of metabolites in the mutant were not so much different from those in the wild type under photoautotrophic conditions. The low enzymatic activity in the OPP pathway in the mutant could not be rate-limiting under photoautotrophic conditions, since the carbon flow to the OPP pathway is largely restricted due to low availability of substrates. Without conflicting with the activity of the OPP pathway, the higher activity of the Calvin cycle in the mutant could bring about a higher growth rate under photoautotrophic conditions (Hihara et al., 1998). Apparently, a delicate balancing between anabolic and catabolic activities is indispensable for growth specifically under photomixotrophic conditions where both light and glucose are available. PmgA is likely to be involved in this regulation by partitioning the carbon flow between the Calvin cycle and the OPP pathway.
Acknowledgments
This work was supported by a Grant-in-Aid for Young Scientists of the Japan Society for the Promotion of Science and a grant from the Ministry of Agriculture, Forestry and Fishery, Japan and CREST, JST, Japan.
Glossary
Abbreviations
- CE/MS
capillary electrophoresis mass spectrometry
- DHAP
dihydroxyacetone phosphate
- FBP
fructose-1,6-bisphosphate
- GA3P
glyceraldehyde-3-phosphate
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- G6P
glucose-6-phosphate
- G6PDH
glucose-6-phosphate dehydrogenase
- ICDH
isocitrate dehydrogenase
- 2-OG
2-oxoglutarate
- OPP pathway
oxidative pentose phosphate pathway
- PEP
phosphoenolpyruvate
- 3PGA
3-phosphoglycerate
- 6PG
6-phosphogluconate
- 6PGDH
6-phosphogluconate dehydrogenase
- PRK
phosphoribulokinase
- R5P
ribose-5-phosphate
- Ru5P
ribulose-5-phosphate
- RuBP
ribulose-1,5-diphosphate
- TCA cycle
tricarboxylic acid cycle
References
- Cooley JW, Vermaas WFJ. Succinate dehydrogenase and other respiratory pathways in thylakoid membranes of Synechocystis sp.strain PCC 6803: capacity comparisons and physiological function. Journal of Bacteriology. 2001;183:4251–4258. doi: 10.1128/JB.183.14.4251-4258.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill RT, Katsoulakis E, Schmitt W, Taroncher-Oldenburg G, Misra J, Stephanopoulos G. Genome-wide dynamic transcriptional profiling of the light-to-dark transition in Synechocystis sp. strain PCC 6803. Journal of Bacteriology. 2002;184:3671–3681. doi: 10.1128/JB.184.13.3671-3681.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranen M, Battchikova N, Zhang P, Graf A, Sirpio S, Paakkarinen V, Aro EM. Towards functional proteomics of membrane protein complexes in Synechocystis sp. PCC 6803. Plant Physiology. 2004;134:470–481. doi: 10.1104/pp.103.032326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hihara Y, Ikeuchi M. Mutation in a novel gene required for photomixotrophic growth leads to enhanced photoautotrophic growth of Synechocystis sp. PCC 6803. Photosynthesis Research. 1997;53:243–252. [Google Scholar]
- Hihara Y, Sonoike K, Ikeuchi M. A novel gene, pmgA, specifically regulates photosystem stoichiometry in the cyanobacterium Synechocystis sp. PCC 6803 in response to high light. Plant Physiology. 1998;117:1205–1216. doi: 10.1104/pp.117.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlon S, Beeri K, Ohkawa H, Hihara Y, Murik O, Suzuki I, Ogawa T, Kaplan A. A putative sensor kinase, Hik31, is involved in the response of Synechocystis sp. strain PCC 6803 to the presence of glucose. Microbiology. 2006;152:647–655. doi: 10.1099/mic.0.28510-0. [DOI] [PubMed] [Google Scholar]
- Knowles VL, Plaxton WC. From genome to enzyme: analysis of key glycolytic and oxidative pentose-phosphate pathway enzymes in the cyanobacterium Synechocystis sp. PCC 6803. Plant and Cell Physiology. 2003;44:758–763. doi: 10.1093/pcp/pcg086. [DOI] [PubMed] [Google Scholar]
- Kurian D, Jansen T, Maenpaa P. Proteomic analysis of heterotrophy in Synechocystis sp. PCC 6803. Proteomics. 2006;6:1483–1494. doi: 10.1002/pmic.200500413. [DOI] [PubMed] [Google Scholar]
- Muro-Pastor MI, Reyes JC, Florencio FJ. Cyanobacteria perceive nitrogen status by sensing intracellular 2-oxoglutarate levels. Journal of Biological Chemistry. 2001;276:38320–38328. doi: 10.1074/jbc.M105297200. [DOI] [PubMed] [Google Scholar]
- Pearce J, Leach CK, Carr NG. The incomplete tricarboxylic acid cycle in the blue-green alga Anabaena variabilis. Journal of General Microbiology. 1969;55:371–378. doi: 10.1099/00221287-55-3-371. [DOI] [PubMed] [Google Scholar]
- Pelroy RA, Rippka R, Stanier RY. Metabolism of glucose by unicellular blue-green algae. Archiv für Mikrobiologie. 1972;87:303–322. doi: 10.1007/BF00409131. [DOI] [PubMed] [Google Scholar]
- Rippka R, Deruelles J, Waterbury J-B, Herdman M, Stanier RY. Genetic assignments, strain histories and properties of pure cultures of cyanobacteria. Journal of General Microbiology. 1979;111:1–61. [Google Scholar]
- Sakuragi Y, Maeda H, DellaPenna D, Bryant DA. α-Tocopherol plays a role in photosynthesis and macronutrient homeostasis of the cyanobacterium Synechocystis sp. PCC 6803 that is independent of its antioxidant function. Plant Physiology. 2006;141:508–521. doi: 10.1104/pp.105.074765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Soga T, Nishioka T, Tomita M. Simultaneous determination of the main metabolites in rice leaves using capillary electrophoresis mass spectrometry and capillary electrophoresis diode array detection. The Plant Journal. 2004;40:151–163. doi: 10.1111/j.1365-313X.2004.02187.x. [DOI] [PubMed] [Google Scholar]
- Singh AK, Sherman LA. Pleiotropic effect of a histidine kinase on carbohydrate metabolism in Synechocystis sp. strain PCC 6803 and its requirement for heterotrophic growth. Journal of Bacteriology. 2005;187:2368–2376. doi: 10.1128/JB.187.7.2368-2376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ. Modes of cyanobacterial carbon metabolism. In: Carr NG, Whitton BA, editors. The biology of cyanobacteria. Berkeley, CA: University of California Press; 1982. pp. 47–85. [Google Scholar]
- Soga T, Ueno Y, Naraoka H, Ohashi Y, Tomita M, Nishioka T. Simultaneous determination of anionic intermediates for Bacillus subtilis metabolic pathways by capillary electrophoresis electrospray ionization mass spectrometry. Analytical Chemistry. 2002;74:2233–2239. doi: 10.1021/ac020064n. [DOI] [PubMed] [Google Scholar]
- Stal LJ, Moezelaar R. Fermentation in cyanobacteria. FEMS Microbiology Reviews. 1997;21:179–211. [Google Scholar]
- Takahashi H, Hayashi M, Goto F, Sato S, Soga S, Nishioka T, Tomita M, Kawai-Yamada M, Uchimiya H. Evaluation of metabolic alteration in transgenic rice overexpressing dihydroflavonol-4-reductase. Annals of Botany. 2006a;98:819–825. doi: 10.1093/aob/mcl162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Watanabe A, Tanaka A, Hashida S-N, Kawai-Yamada M, Sonoike K, Uchimiya H. Chloroplast NAD kinase is essential for energy transduction through the xanthophyll cycle in photosynthesis. Plant and Cell Physiology. 2006b;47:1678–1682. doi: 10.1093/pcp/pcl029. [DOI] [PubMed] [Google Scholar]
- Tamoi M, Miyazaki T, Fukamizo T, Shigeoka S. The Calvin cycle in cyaobacteria is regulated by CP12 via the NAD(H)/NADP(H) ratio under light/dark conditions. The Plant Journal. 2005;42:504–513. doi: 10.1111/j.1365-313X.2005.02391.x. [DOI] [PubMed] [Google Scholar]
- Trentham DR, McMurray CH, Pogson CI. The active chemical state of D-glyceraldehyde 3-phosphate in its reactions with D-glyceraldehyde 3-phosphate dehydrogenase, aldolase and triose phosphate isomerase. Biochemical Journal. 1969;114:19–24. doi: 10.1042/bj1140019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedel N, Soll J. Evolutionary conserved light regulation of Calvin cycle activity by NADPH-mediated reversible phosphoribulokinase/CP12/ glyceraldehyde-3-phosphate dehydrogenase complex dissociation. Proceedings of the National Academy of Sciences, USA. 1998;95:9699–9704. doi: 10.1073/pnas.95.16.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Hua Q, Shimizu K. Integration of the information from gene expression and metabolic fluxes for the analysis of the regulatory mechanisms in Synechocystis. Applied Microbiology and Biotechnology. 2002a;58:813–822. doi: 10.1007/s00253-002-0949-0. [DOI] [PubMed] [Google Scholar]
- Yang C, Hua Q, Shimizu K. Metabolic flux analysis in Synechocystis using isotope distribution from 13C-labeled glucose. Metabolic Engineering. 2002b;4:202–216. doi: 10.1006/mben.2002.0226. [DOI] [PubMed] [Google Scholar]
- Yang C, Hua Q, Shimizu K. Quantitative analysis of intracellular metabolic fluxes using GC-MS and two-dimensional NMR spectroscopy. Journal of Bioscience and Bioengineering. 2002c;93:78–87. [PubMed] [Google Scholar]