Abstract
The structure and transport properties of pit membranes at the interface between the metaxylem and xylem parenchyma cells and the possible role of these pit membranes in solute transfer to the phloem were investigated. Electron microscopy revealed a fibrillar, almost tubular matrix within the pit membrane structure between the xylem vessels and xylem parenchyma of leaf blade bundles in rice (Oryza sativa). These pits are involved primarily with regulating water flux to the surrounding xylem parenchyma cells. Vascular parenchyma cells contain large mitochondrial populations, numerous dictyosomes, endomembrane complexes, and vesicles in close proximity to the pit membrane. Taken collectively, this suggests that endocytosis may occur at this interface. A weak solution of 5,6-carboxyfluorescein diacetate (5,6-CFDA) was applied to cut ends of leaves and, after a minimum of 30 min, the distribution of the fluorescent cleavage product, 5,6-carboxyfluorescein (5,6-CF), was observed using confocal microscopy. Cleavage of 5,6-CFDA occurred within the xylem parenchyma cells, and the non-polar 5,6-CF was then symplasmically transported to other parenchyma elements and ultimately, via numerous pore plasmodesmata, to adjacent thick-walled sieve tubes. Application of Lucifer Yellow, and, separately, Texas Red-labelled dextran (10 kDa) to the transpiration stream, confirmed that these membrane-impermeant probes could only have been offloaded from the xylem via the xylem vessel–xylem parenchyma pit membranes, suggesting endocytotic transmembrane transfer of these membrane-impermeant fluorophores. Accumulation within the thick-walled sieve tubes, but not in thin-walled sieve tubes, confirms the presence of a symplasmic phloem loading pathway, via pore plasmodesmata between xylem parenchyma and thick-walled sieve tubes, but not thin-walled sieve tubes.
Keywords: Apoplasmic and symplasmic transport, companion cell, exo- and endocytosis, Oryza sativa, pit membrane, rice, thick-walled sieve tube, thin-walled sieve tube, xylem parenchyma
Introduction
Vascular bundles in leaves are compact structures, and xylem and phloem occur in close proximity to each other. The transport of assimilates to the phloem and the recycling of water from the xylem in plant leaves are complex processes (see Canny, 1988, 1993; Evert et al., 1978, and references therein) and most, if not all, of the water entering source phloem may be taken up directly from the transpiration stream as a direct consequence of increased solute concentration within the loading sieve tubes. Transfer from the xylem involves pit membranes situated between tracheary elements and associated parenchyma cells. These pit membranes are characterized by an additional wall layer, deposited next to the plasma membrane of the xylem parenchyma cell abutting xylem vessels. The pit membrane is variously described in the literature as a ‘protection’ or ‘additional layer’ (Shaffer and Wisniewski, 1989), which is involved in the control of hydraulic resistance (Zwieniecki et al., 2001). Various free space markers used in the past have demonstrated that water and tracer ions can move from xylem vessels (see Wisniewski et al., 1991) into xylem parenchyma via interconnecting pits, and, more importantly, it has been demonstrated that these tracers end up in the phloem apoplast (Evert et al., 1985; Botha and Evert, 1986; Botha, 2006). Van Bel (1990) noted that transfer from the xylem to the phloem involved several steps including passage across this interface and ultimately uptake by the phloem.
The rice leaf blade, like many other monocotyledonous leaf blades examined to date, contains three longitudinal vein orders. All three (large, intermediate, and small) contain thin- as well as thick-walled sieve tubes which were not previously reported by Chonan and co-workers in their rice leaf blade studies (Chonan et al., 1980, 1981, 1984, 1985). The thick-walled sieve tubes, which are either in direct contact with or in close proximity to metaxylem vessels, may have functions different from those of the thin-walled sieve tubes (Botha and Evert, 1986; Evert et al., 1978, and references therein). The predominant, early metaphloem is composed of thin-walled sieve tube members and associated companion cells, as well as parenchymatous elements. In contrast, thick-walled, late-formed metaphloem sieve tube members lack companion cells and appear to be symplasmically isolated from the thin-walled sieve tube–companion cell complexes. Fossil data suggest that thick-walled sieve tubes have been present in the phloem of grasses for >5 million years (see images in Thomasson et al., 1986). This persistence during such a long period of evolution implies a likely functional role which has so far eluded researchers. For example, Heyser et al. (1978) reported the presence of a tubular substructure in the walls of the thick-walled sieve elements in maize leaves which they felt could be implicated in solute retrieval processes. Recently, Matsiliza and Botha (2002) and Botha and Matsiliza (2004) demonstrated that aphids do not preferentially feed from thick-walled sieve tubes in barley leaves. Evidence from experiments involving retrieval of [14C]sucrose (Heyser et al., 1978) as well as the distribution of cleaved polar molecules such as 5,6-carboxyfluorescein (5,6-CF) suggest that solute retrieval from the xylem could involve thin- as well as thick-walled sieve tubes.
It has been demonstrated in previous studies that the application of a weak solution of 5,6-carboxyfluorescein diacetate (5,6-CFDA) for 10–30 min to the transpiration stream resulted in the appearance of 5,6-CF in cells outside the tracheary elements of vascular bundles in the leaves, some distance from the point of application. Even though 5,6-CFDA could diffuse apoplasmically across cell walls and then enter plant cells, this is unlikely as the first point in the transport pathway where 5,6-CF was detected was always associated with the xylem vessel–xylem parenchyma interface, in the pit membrane regions. These results suggest a localized zone of xylem vessel to xylem parenchyma transfer in leaves (Botha, 2006). Cleavage of 5,6-CFDA requires metabolic activity, and thus could occur only via esterase activity within the living cells abutting metaxylem vessels in these bundles. After cleavage, the highly polar 5,6-CF molecule is not able to cross cell membranes, and cell to cell transport is then symplastic, via plasmodesmata (Wright et al., 1996; Botha, 2006, and relevant literature cited therein).
The focus of this study includes the pit membranes which form the boundary between the apoplast (xylem vessels) and the symplast (xylem parenchyma) in Oryza sativa as in other grass species (Botha, 2006). These pits, and their associated membranes, are considered to be a vital physiological barrier (Zwieniecki et al., 2001) between the apoplasmic and symplasmic compartments. The transmission electron microscopy (TEM) and transport study reported here focuses on the following specific questions:
Are the xylem vessel–xylem parenchyma pits structurally significant in terms of facilitating solute exchange across this interface?
Do xylem parenchyma cells have the capacity to take up 5,6-CFDA and cleave the diacetate to form membrane-impermeable 5,6-CF?
If so, what pathways are involved in the movement of 5,6-CF from xylem parenchyma cells?
Can it be demonstrated that membrane-impermeant phloem-mobile, symplasmically transported fluorophores, such as 5,6-CF, Texas Red–dextran (TRD) and Lucifer Yellow (LYCH), are transported via the xylem, offloaded into vascular parenchyma elements, and subsequently taken up by thin-walled and/or thick-walled sieve tubes?
Does the xylem vessel–xylem parenchyma interface provide a solute retrieval pathway?
Materials and methods
Plant material
Rice plants of O. sativa L. cv. Nipponbare were grown to maturity in flooded paddy tanks in a glasshouse, in pots containing 75% compost and 25% perlite supplemented with Osmocote slow-release fertilizer. Growth was under natural light with a daytime temperature of 28 °C and a night-time temperature of 25 °C. Mature leaves from pre- or post-flowering plants were used in experiments.
Transmission electron microscopy (TEM)
Segments from the mid-lamina region of mature vegetative leaves were cut from O. sativa cv. Nipponbare plants directly into cold primary fixative (composed of 2.5% glutaraldehyde and 0.5% paraformaldehyde in 0.1 M sodium cacodylate buffer), and immediately placed in vials containing the fixative in an ice bucket. After 30 min, leaf segments were carefully trimmed to remove edge material damaged during initial specimen preparation. Retrimmed segments were returned to fresh primary fixative. Trimmed segments were fixed in several changes of the primary fixative overnight at 4 °C. After several buffer rinses, the leaf tissue was post-fixed in 1% OsO4 in 0.1 M sodium cacodylate buffer, left overnight at 4 °C, subsequently dehydrated through a cold ethanol series, followed by several changes of propylene oxide, and embedded in Spurr's resin (hard grade). Ultrathin sections (silver) were stained with uranyl acetate and lead citrate before TEM observation using a JEOL 1210 (Tokyo, Japan) electron microscope. High resolution analogue images were taken on cut film, and selected regions were digitized directly from the negatives using a scanner at 2400×2400 dpi.
Fluorescent probes
A 188 mM stock solution of 5,6-CFDA (Sigma C8166) was prepared in dimethylsulphoxide (DMSO) and was diluted with distilled water to provide a working solution with a final concentration of 0.188 mM. A 40 mM stock solution of sulphorhodamine-101 HCl [Texas Red (TR) Sigma, S3388] was made up in DMSO and diluted with distilled water to a final concentration of 0.04 mM. In dual-probe experiments, 500 μl of this working strength solution was diluted further by adding 500 μl of distilled water. A 54.7 mM solution of Lucifer Yellow CH (LYCH; dilithium salt Sigma-Aldrich, L0259) was prepared in distilled water and diluted to a 0.547 mM working solution. An aqueous stock solution of 2.5 mM TRD (D1828 neutral, molecular weight 10 000 Da, Molecular Probes) was diluted to a working strength of 0.156 mM in distilled water. Stock and working strength solutions were kept foil-wrapped and stored at –4 °C until needed. Prior to use, the working strength probes were centrifuged at 14 000 g for 15 min, using Microcon 3000 Da or 10 000 Da microconcentrators (Amicon Inc., Beverly, MA, USA) to ensure removal of free fluorochromes.
Fluorescent tracer analysis
Fully expanded source (assimilate exporting) leaves were rapidly cut from pre- and post-flowering rice, and immediately transferred into a beaker containing water. As needed, excised leaves were transferred directly into a mixture usually containing equal volumes of working strength 5,6-CFDA and TR. The fluorophore 5,6-CFDA was co-transported with TR. TR is transported in the xylem and remains principally attached to lignified cell walls; it thus revealed the position of the translocating xylem very effectively. Non-polar, non-fluorescent 5,6-CFDA is cleaved only within living cells to form highly fluorescent, strongly polar 5,6-CF once it exits the tracheary elements.
Cut leaves, with their cut ends in dye solution, were illuminated (∼150 μmol m−2 s−1) using a small 15 W table lamp. Experiments were run for a minimum of 30 min, after which time the position of the 5,6-CF fluorescence front was detected using a green fluorescent protein (GFP) filter set on a Leica MZFLIII or MZ6 stereo fluorescence microscope, and its location carefully marked on the edge of the leaves using a permanent fine point marker pen. Under the experimental conditions described, the fluorescence front was usually 10 cm or more from the cut base of the leaf. The spread of 5,6-CF was monitored in more detail by examining intact leaf tissue and tissue sections taken progressively further from the cut leaf base using an Olympus BX-51 (Olympus, Wirsam Scientific, Johannesburg, South Africa) widefield fluorescence microscope, and multiple fluorescence (m-FIP) images were captured using Analysis 5 (Olympus, Tokyo Japan) imaging software and either a high-resolution F-View, or colourview FX Peltier cooled camera (Olympus, Wirsam Scientific, Johannesburg, South Africa). In all cases, fluorescent probes were detected only in the longitudinal or cross-veins, except in the immediate vicinity of the cut base of the leaf.
Due to the inherent difficulties associated with cutting longitudinal sections of living tissue and still retaining cell integrity, thick (50–200 μm) transverse sections were routinely prepared. Leaves were examined not less than 10 cm from the cut end of the leaf to ensure that transport in undamaged tissue was observed. Using single-edge razor blades, transverse sections were cut directly into silicone oils (to minimize loss of the fluorophore from exposed cells) and transferred directly to microscope slides. Sections were mounted in silicone oil under coverslips and examined immediately using a Leica TCS SP2 confocal laser scanning microscope (CLSM). The 5,6-CF was excited by the 488 nm line from an argon–ion laser, whilst TR was excited with the 543 nm line from a He–Ne laser. Fluorescence emission was collected over 500–530 nm for 5,6-CF, 610–635 nm for TR, and 680–720 nm for chlorophyll autofluorescence. In experiments using LYCH, fluorescence emission was collected over 500–580 nm following excitation at 405 nm. The confocal images presented here are representative of many replicate experiments.
Results
Ultrastructure of transport tissues
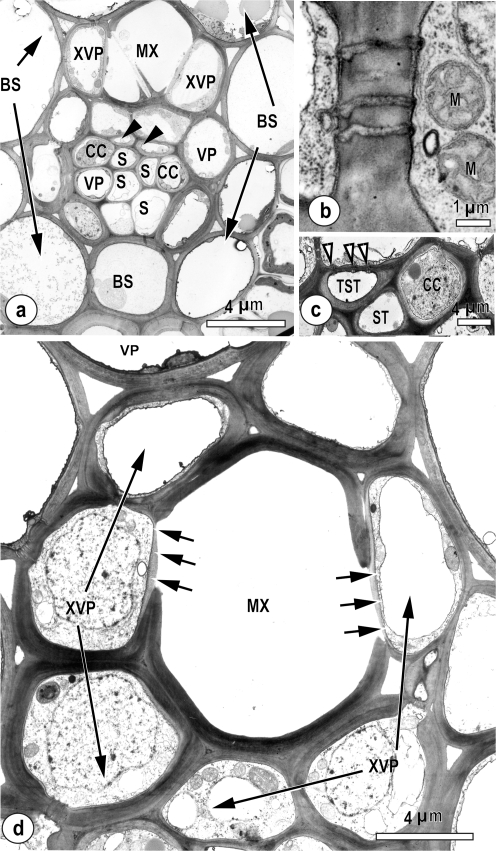
The xylem of the intermediate leaf blade vascular bundles is composed of metaxylem vessels and parenchyma (Fig. 1a–d), and the phloem contains two types of sieve tube—early metaphloem (S; described as thin-walled), associated with companion cells (CC), and late-formed metaphloem (described as thick-walled; arrowheads in Fig. 1a indicate two thick-walled sieve tubes). These thick-walled sieve tubes lacked companion cells (Fig. 1a, c), but were in symplasmic contact with vascular parenchyma (details of pore-plasmodesmata not shown). Xylem vascular parenchyma (XVP; Fig. 1a, d) was connected via plasmodesmata (Fig. 1b) to other vascular parenchyma (VP) and by pore-plasmodesmata (paired arrowheads, Fig. 1c) to thick-walled sieve tubes. Of interest were the thin-walled structures between the metaxylem vessels and XVP (arrows, Fig. 1d). This pit structure has been described elsewhere as a hydrolysed wall (Evert et al. 1978).
Fig. 1.
TEM images, showing details of vascular tissue in intermediate vascular bundles in leaf blades of Oryza sativa. (a) Intermediate vein with metaxylem vessels (MX) and associated xylem vascular parenchyma (XVP). The phloem contains two thick-walled sieve tubes (arrowheads) and thin-walled sieve tubes (S), associated companion cells (CC), and parenchyma cells (VP) surrounded by a bundle sheath (BS). (b) A plasmodesmal field between a xylem vascular parenchyma cell (right) and a parenchyma cell (left). Note mitochondria (M). (c) Detail showing a thick-walled sieve tube (TST) with pore-plasmodesmata connections (arrowheads) to the vascular parenchyma cell above. The thin-walled sieve tube (ST) is associated with a CC and two adjacent xylem parenchyma cells. (d) A small intermediate vascular bundle showing hydrolysed cell wall (arrows) between the metaxylem vessel (MX) and associated xylem parenchyma (XVP) which has a loosely fibrillar appearance. The wall structure between the xylem vessel and the xylem parenchyma is highly modified, hydrolysed on the vessel side, and delimited by a plasma membrane on the parenchyma cell side.
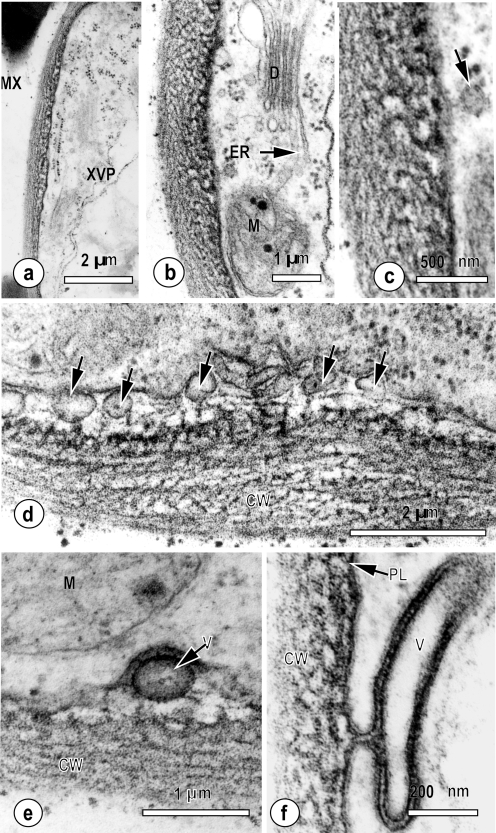
In a more detailed examination of the pit region between metaxylem vessels and the xylem parenchyma (Fig. 2a–f), the wall appeared porous, with a complex substructure (Fig. 2a, b). Furthermore, the inner face of the wall (nearest the vascular parenchyma) appeared more electron-dense than the outer face associated with the metaxylem vessel. This interface contained numerous vesicles (short arrows, Fig. 2d) between the parenchyma cell plasma membrane and the hydrolysed pit membrane. Stalked vesicles (Fig. 2c, arrow) were appressed to the outer plasma membrane leaflet, and were apparently encapsulated by a double unit membrane (V, Fig. 2e). Occasionally, large, distinctly granular plasma membrane blebs (Fig. 2f) were observed in the pit membrane region. Pit-containing xylem parenchyma cells always contained large mitochondrial populations (M; Fig. 2a, b), which were, in turn, closely associated with dictyosomes and endoplasmic reticulum (D and ER respectively, Fig. 2b). Although not quantified, all vascular parenchyma cells examined were well connected by plasmodesmata (Fig. 1b), which is in agreement with previous studies of monocotyledonous leaves (Botha, 2006). When examined at higher magnification, the wall structure in the pit regions appeared sponge-like with numerous narrow tubular substructures (Fig. 2c–e).
Fig. 2.
Ultrastructural detail of the hydrolysed pit membrane typically observed between the metaxylem vessels and xylem parenchyma elements in small, intermediate, and large veins in leaf blades of Oryza sativa. (a) Pit membrane between a tracheary element (TE) and xylem parenchyma cell (XVP) which has a perforate, fenestrated appearance. Numerous mitochondria (M) are found in XVP cells. (b) Pit membrane of xylem parenchyma cell with adjacent mitochondrion (M), endoplasmic reticulum (ER), and dictyosome (D). (c–f) Details of the wall interface showing the vesicles at the membrane–wall interface. (c) Stalked vesicle attached to the plasma membrane. (d) Spherical vesicles (solid arrows) occur beneath the plasma membrane, attached to the outer face of the hydrolysed wall region by thin electron-dense stalks. The plasma membrane adjacent to the cell wall is elaborated with interspersed tubules (arrowheads). (e) The plasma membrane appears discontinuous, especially adjacent to vesicles (V) which appear to have a double membrane. (f) Detail of an elongate vesicle (V) attached to the plasma membrane (PL) attached to and in close association with the underlying hydrolysed pit membrane region of the cell wall (CW).
Dye uptake and transport
Previous work showed rapid movement of 5,6-CFDA through the xylem and rapid appearance of its cleavage product, 5,6-CF, within leaf vascular parenchyma (Botha, 2006). This observation and the TEM study described above led to examination of the potential transport pathway from xylem vessels to xylem parenchyma cells in more detail.
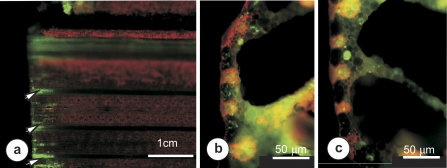
Upon supplying 5,6-CFDA to the transpiration stream, it was first determined that within 30 min, the 5,6-CF produced by cleavage of 5,6-CFDA at the cut surface of the leaf diffused no more than 1 cm through basal leaf tissues (Fig. 3a). Close to the cut surface, cross-sections showed a wide distribution of 5,6-CF throughout all leaf tissues (Fig. 3b); however, as close as 1 mm from the surface (Fig. 3c), the dye was largely restricted to living cells, indicating diffusion of dye cleaved by esterases within living cells rather than diffusion of cleaved dye in the external solution and apoplast. A more detailed examination of single vascular bundles showed TR in cell walls of xylem vessels and vascular parenchyma 20 cm from the cut surface 30 min after supply to the cut end of the leaf (Fig. 4). In contrast, 5,6-CF was seen only in the cytoplasm of vascular parenchyma at 10 cm from the cut surface, and was absent from the phloem by 3 cm from the cut. It was concluded that 5,6-CF seen in living cells of leaf cross-sections taken at least 10 cm from the cut surface of the leaf represented intracellular cleavage of intact 5,6-CFDA supplied by the xylem.
Fig. 3.
Widefield fluorescence micrographs illustrating 5,6-CF transport within cut leaves. (a) The basal 5 cm of the leaf 30 min after placing in 5,6-CFDA solution. Wide distribution of 5,6-CF was seen only in tissues within 1 cm of the severed end of the leaf (arrows). (b) A transverse section of the midrib region, cut in the basal 300 μm of the severed leaf, showing abundant 5,6-CF throughout leaf tissues. (c) Transection of the midrib cut ∼1000 μm from the severed end of the leaf showing 5,6-CF present only within cells of the mesophyll and vascular parenchyma close to the xylem.
Fig. 4.
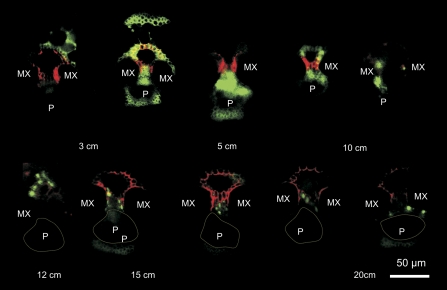
Widefield fluorescence micrographs of thick sections taken between 3 cm and 20 cm from the cut leaf base 30 min after placing in a solution of 5,6-CFDA and TR. TR fluorescence was detected in metaxylem (MX) and vascular parenchyma cell walls in all sections. At 3 cm and 5 cm, 5,6-CF fluorescence is detected in all cells of the vascular bundle and in cell walls of the surrounding sheath tissue. Between 10 cm and 20 cm, 5,6-CF fluorescence was restricted largely to vascular parenchyma around the xylem vessels, with no fluorescence detectable in the phloem (P, dotted line approximates the position of the phloem tissue in the vascular bundles). At 20 cm, 5,6-CF fluorescence was limited to a few xylem parenchyma cells abutting the metaxylem vessels.
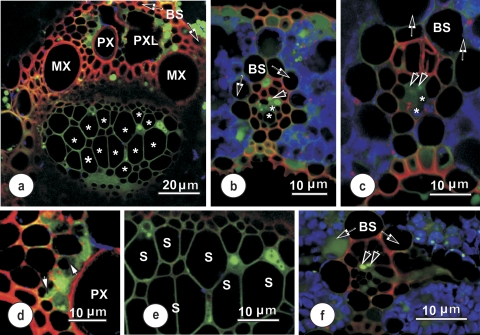
Confocal images (Fig. 5) confirmed that the localization of 5,6-CF was clearly intracellular, and associated with living cells only (this is most clearly demonstrated in Fig. 5a). Some parenchymatous elements adjacent to the protoxylem lacuna contained 5,6-CF (Fig. 5d), but there was no evidence of back-leakage of the fluorophore into the protoxylem cavity. 5,6-CF fluorescence was never observed within xylem vessels, in either large (Fig. 5a, d), intermediate (Fig. 5b, c), or small (Fig. 5f) vascular bundles. The fluorophore only occasionally appeared in sieve tubes. Phloem parenchyma cells also contained 5,6-CF (see Fig. 5e for detail), but none was evident in the companion cells or the metaphloem sieve tubes (S) in images from this series of sections. Of note was that 5,6-CF was taken up by the thick-walled sieve tubes (paired arrowheads, Fig. 5c, f) and transported laterally to a connected thin-walled sieve tube (TST) within the transverse vein. Some 5,6-CF fluorescence was seen occasionally outside the vascular bundle (Fig. 5b). Given that there are plasmodesmata along the interfaces from the vascular parenchyma through the bundle sheath and into the mesophyll, this result is not surprising as water diffuses rapidly and presumably can make use of the symplasmic pathway that exists from vascular parenchyma to the mesophyll. It is clear that the results presented here are in line with earlier findings (Botha, 2006; Wright et al., 1996) that cleavage of 5,6-CFDA occurs within the living (symplasmic), rather than the non-living (apoplasmic) domain.
Fig. 5.
Confocal laser scanning micrographs showing the distribution of 5,6-CF (green) and TR (red) in vascular bundles 10–20 cm from the base of a rice leaf, after supplying with 5,6-CDFA together with TR for 60–90 min. Blue coloration indicates chloroplast autofluorescence. (a) Large vascular bundle, showing 5,6-CF and TR distribution. 5,6-CF is localized in parenchyma adjacent to the protoxylem (PX) and some in xylem parenchyma on the phloem side of this vascular bundle. The lumina of large (thin-walled) metaphloem sieve tubes (stars) showed no evidence of 5,6-CF, but the fluorophore appears in all cell walls of the phloem in this section. (b and c) Intermediate longitudinal veins, showing accumulation of 5,6-CF in vascular parenchyma cells. Note: some fluorophore has moved out into mesophyll cells. 5,6-CF is seen in parenchyma elements in the phloem, accumulated in thick-walled (paired arrowheads), but not in the large thin-walled sieve tubes (asterisks). (d) Detail showing the distribution of 5,6-CF in xylem parenchyma adjacent to a protoxylem vessel. Despite some evidence of 5,6-CF in cytoplasm and vacuoles, it was confined to the cytoplasm (arrowheads) one cell removed from the xylem–xylem parenchyma interface. The probe was absent from the protoxylem vessel. (e) Detail of the phloem from (a). Here, 5,6-CF is seen only in phloem parenchyma. Sieve tubes (S) and associated companion cells in this and other undamaged sections contained no 5,6-CF. (f) Transection of a small longitudinal vein, surrounded by a bundle sheath (BS). An emergent thick-walled sieve tube (paired arrowheads point to two TSTs which contain 5,6-CF) in a connecting transverse vein is visible to the right. The TSTs connect to a thick-walled sieve tube exiting from the longitudinal bundle to the right.
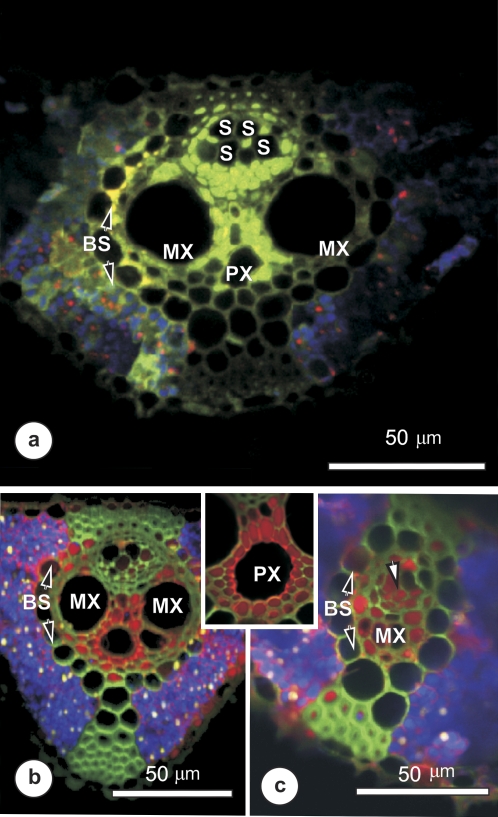
The uptake and distribution of a relatively small (LYCH) or large (10 kDa TRD) apoplasmic fluorophore was assessed to make a comparison with the symplastic movement of 5,6-CF. It was assumed that these dyes would differ mainly in the extent of their migration through the apoplast. Figure 6 shows that LYCH (Fig. 6a) was taken up into the symplast of xylem parenchyma around the metaxylem (MX) and protoxylem (PX), and spread throughout the vascular parenchyma and thick-walled sieve tubes (S). The TRD spread rapidly from the MX (Fig 6a, b) and PX (inset) in both large (Fig. 6b) and small (Fig. 6c) intermediate veins. The dye was detected throughout vascular parenchyma and in thick-walled sieve tubes and, as illustrated here, within some thin-walled metaphloem sieve tubes (arrow in Fig. 6c). Neither LYCH nor TRD was detected in any xylem vessels.
Fig. 6.
Confocal laser scanning micrographs, showing the distribution of LYCH (yellow-green) or 10 kDa TRD (red) in vascular bundles 10–20 cm from the base of a rice leaf, after supplying with either LYCH (a) or TRD (b and c) for 60–90 min. Blue coloration indicates chloroplast autofluorescence. (a) Cross-section of a large vascular bundle showing LYCH in xylem parenchyma between the large metaxylem vessels (MX), in phloem parenchyma, and traces in some large-diameter metaphloem elements (S). Strongly fluorescent patches were seen near the bundle sheath cells (BS), and some fluorescence was seen outside the vascular bundle. (b and c) Distribution of TRD within a large (b) and a small (c) intermediate vein. The xylem parenchyma was very strongly labelled, suggesting rapid offloading to the parenchyma from xylem vessels. TRD spread from the metaxylem (MX) as well as the protoxylem (inset, PX) to xylem parenchyma and phloem-associated parenchyma, as well as thick-walled sieve tubes and some thin-walled sieve tubes (arrow in c).
Discussion
Structural significance of the pit membranes
This study, in part, addresses a fundamental question in plant water relations physiology: what is the fate of material transported passively in the xylem? Clearly, these substances must be retrieved and recycled for distribution to regions where they are required. The current TEM study shows that the major interface between xylem vessels and associated xylem parenchyma is the pit membrane. At this interface, the fenestrated xylem vessel wall with its complex compartmented membrane structure and associated membrane-bound vesicles (Fig. 5a–f) suggests an active transmembrane transport process other than simple diffusion. Vesiculation in close proximity to dictyosomes, together with large mitochondrial populations, suggest that this interface may play an important physiological role in apoplast to symplast transfer, which could be regulated through ion-mediated and dynamic changes in hydrogels (Zwieniecki et al., 2001). LYCH crossed the interface between the xylem vessel and xylem parenchyma, implying that active membrane transport may be involved. LYCH has previously been shown to enter the vacuole of a range of plant cell protoplasts from the external medium (Wright and Oparka, 1989). That LYCH as well as TRD could cross the interface between the xylem vessels and xylem parenchyma is good evidence for a complex interchange process (endo- and/or exocytosis) between the apoplast and the symplast in the rice leaf.
Which cells are involved in transfer from the apoplasm to the symplasm?
It has been demonstrated, using CLSM, that 5,6-CFDA, LYCH, and TRD can exit the xylem and that, in the case of 5,6-CFDA, its polar fluorescent cleavage product, 5,6-CF, appears first in the xylem parenchyma cells abutting the xylem vessels (Figs 3–6). Beyond the zone of damaged cells at the cut base of the leaf (500 μm to no more than 1000 μm, Fig. 3), the xylem parenchyma are the first receiver cells for cleaved 5,6-CF (Fig. 4). Examination of whole leaf material confirmed that fluorescent areas were discrete and sometimes patchy in distribution (data not shown), suggesting that the 5,6-CF was trafficked symplasmically from discrete locations rather than simply leaking from the xylem. Taken together, the data presented here strongly support a localized pathway for retrieval of materials from the xylem (apoplasm) into the living parenchyma cells (symplasm) via pit membranes, after which the fluorescent, highly polar 5,6-CF can be transported away from the xylem parenchyma. Lack of diffuse fluorescence across multiple cell types of the leaf after 5,6-CFDA feeding indicates that lateral diffusion of the uncleaved dye followed by later cleavage in cells distant from xylem parenchyma was unlikely in these experiments. The same conclusion was reached by previous groups using this dye (see discussion in Wright et al., 1989, 1996).
Data for LYCH and TRD transport suggest that the highly complex pit membrane region may be a site of uptake from xylem apoplast to xylem parenchyma symplast, via transfer across the permeable cell wall and parenchyma plasma membrane. The membrane vesiculation in this region suggests a role for endocytosis in uptake of these normally membrane-impermeant dyes. Earlier TEM analysis demonstrated a distinct export pathway of lanthanum and lead nitrate, as well as Prussian blue, from the transpiration stream to the mesophyll (Botha and Evert, 1986, and references therein). Larger tracers, such as the TRD used here, are not usually transported readily across cell walls; thus the loose cell wall structure of the pit membrane seen in the TEM images probably reflects a high capacity for transfer across this wall. Hence, the xylem parenchyma and specialized pit membranes appear to contain specific features for uptake and transfer of small and large solutes from the xylem into the vascular symplasm.
Which pathways are involved in transport of 5,6-CF, LYCH, and TRD from the xylem parenchyma?
Beyond the xylem parenchyma, symplasmic communication occurs through functional plasmodesmata, but grasses have been generally shown to have low connectivity between vascular parenchyma cells and the companion cell–sieve tube (CC–ST) complexes (Botha, 1992, 2006; van Bel, 1993, and references therein), and more than one operational pathway may be involved in cell–cell transport. The confocal images showing movement of these fluorophores from xylem parenchyma through phloem parenchyma to the CC–ST complexes unequivocally demonstrate the presence of a symplasmic pathway connecting these cells. Free space markers, in contrast, remain confined to the apoplasmic pathway, intercellular spaces, and cell walls, and their movement is, for the most part, along lignified walls (Botha and Evert, 1986). Once the fluorophores reach the CC–ST complexes, they may be transported laterally across the leaf via the transverse connecting strands common in cereal leaves (Fig. 5f), and then into surrounding tissues.
Do the fluorophores accumulate in the sieve tubes of rice leaves?
The CLSM study clearly shows fluorescence within a few sieve tubes in vascular bundles (see Fig. 5f). However, the disjunction between vascular parenchyma and the CC–ST (thin-walled ST; Botha, 1992, 2006; Botha and van Bel, 1992) would seem to preclude significant 5,6-CF symplasmic movement and subsequent accumulation in the thin-walled sieve tubes (see Fig. 5e). In contrast, pore-plasmodesmata between vascular parenchyma and thick-walled sieve tubes may provide a pathway for 5,6-CF entry (see Fig. 5f). There may not be a high flux through this transport pathway as dye feeding experiments demonstrate symplasmic connectivity rather than dynamic transport rates. Moreover, the reported low osmotic potential within (Evert et al., 1978), and the lack of interest of aphids in thick-walled sieve tubes (Botha and Matsiliza, 2004), together with the low plasmodesmatal frequencies reported at the interface between vascular parenchyma and either thick-walled sieve tubes or companion cells, is further evidence that this is not a major assimilate accumulation pathway. Nevertheless, the retrieval of 5,6-CF by thick-walled sieve tubes, together with earlier results by Fritz et al. (1983) showing that the thick-walled sieve tubes in leaf blade veins of Zea are involved in [14C]sucrose retrieval by these sieve tubes, demonstrates a functional symplasmic pathway to thick-walled sieve tubes in grasses.
Is there evidence for a retrieval pathway from the xylem to the phloem?
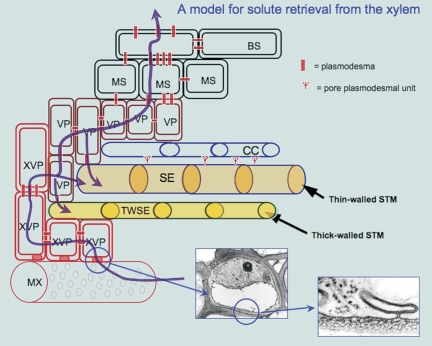
A model of assimilate and solute flow consistent with the data presented here is shown in Fig. 7. Water, ions, and dissolved substances are carried in the xylem and offloaded in transpiring, photosynthesizing leaves, via hydrolysed pit membranes into xylem parenchyma cells. Transfer from the apoplast to the symplast involves passage through the pit membrane between xylem vessels and xylem parenchyma, across the vascular parenchyma cell plasma membrane, where porosity may be enhanced in pectin-rich walls (Baron-Epel et al., 1988). The present TEM and dye transport data provide evidence that this interface is also important in retrieval of larger solutes, possibly via endocytosis. It has been suggested that interface permeability may be regulated by calcium flux, in effect acting to regulate passage of substances, whether secretion via Golgi-related vesicles (Benayoun, 1983) or the control of xylem physiology (Schaffer and Wisniewski, 1989) including changes in diameters of microchannels in the pit membranes (Zwieniecki et al., 2001), or the movement of virus particles through calcium-mediated disruption of the pit membrane (Opalka et al., 1998).
Fig. 7.
Model for retrieval from the apoplast, based upon light, confocal, and TEM studies. Water and dissolved substances are carried in the xylem and are offloaded in transpiring photosynthesizing leaves (pathways shown in purple). This involves transfer from the apoplast to the symplast through the pit membrane between metaxylem vessels and xylem parenchyma (shown in the detailed images at lower right). Vesicles formed between the hydrolysed wall and the plasma membrane may carry other substances that are not directly transferable across the membrane, from the apoplast to the symplast, or vice versa, thus effecting a passively driven exchange process across this interface. BS, bundle sheath; MS, mestome sheath; MX, metaxylem; SE, sieve element; STM, sieve tube membrane; TWSE, thick-walled sieve element; VP, vascular parenchyma; XVP, xylem vascular parenchyma.
Concluding comments
In this study, a pathway for the recovery of material from the apoplast to the symplast in O. sativa leaf blades was demonstrated. It was concluded that all xylem parenchyma cells are potentially involved in this recovery/transport pathway, as demonstrated by their ability to take up membrane-impermeant fluorophores. It is proposed that the pit membranes in rice leaf blade vascular bundles—specifically those at the interface with xylem parenchyma cells—are potentially a major traffic pathway and that they are probably involved in retrieval, regulating resorption and movement across the apoplast–symplast interface of a wide range of material within leaves.
Acknowledgments
We thank Celia Miller for assistance with the preparation of some of the initial samples for TEM. The National Research Foundation Pretoria is acknowledged for grants to CEJB for travel to CSIRO, Canberra, where the confocal study was undertaken.
Glossary
Abbreviations
- 5,6-CFDA
5,6-carboxyfluorescein diacetate
- 5,6-CF
5,6-carboxyfluorescein
- CLSM
confocal laser scanning microscopy
- DMSO
dimethylsulphoxide
- LYCH
Lucifer Yellow carbonyl hydrazine
- TEM
transmission electron microscopy
- TR
Texas Red
- TRD
Texas Red–dextran
References
- Baron-Epel O, Gharyal PK, Schindler M. Pectins as mediators of wall porosity in soybean cells. Planta. 1988;175:389–395. doi: 10.1007/BF00396345. [DOI] [PubMed] [Google Scholar]
- Benayoun J. A cytochemical study of cell wall hydrolysis in the secondary xylem of Poplar (Populis italica Moench) Annals of Botany. 1983;52:189–200. [Google Scholar]
- Botha CEJ. Plasmodesmatal distribution, structure and frequency in relation to assimilation in C3 and C4 grasses in southern Africa. Planta. 1992;187:348–358. doi: 10.1007/BF00195658. [DOI] [PubMed] [Google Scholar]
- Botha CEJ. Interaction of phloem and xylem during phloem loading—functional symplasmic roles for thin- and thick-walled sieve tubes in monocotyledons? In: Holbrook NM, editor. Vascular transport in plants. London: Elsevier; 2006. pp. 115–130. [Google Scholar]
- Botha CEJ, Evert RF. Free-space marker studies on the leaves of Saccharum officinarum and Bromus unioloides. South African Journal of Botany. 1986;52:335–342. [Google Scholar]
- Botha CEJ, Matsiliza B. Reduction in transport in wheat (Triticum aestivum) is caused by sustained phloem feeding by the Russian wheat aphid (Diuraphis noxia) South African Journal of Botany. 2004;70:249–254. [Google Scholar]
- Botha CEJ, van Bel AJE. Quantification of symplastic continuity as visualised by plasmodesmograms: diagnostic value for phloem-loading pathways. Planta. 1992;187:359–366. doi: 10.1007/BF00195659. [DOI] [PubMed] [Google Scholar]
- Canny MJ. Water pathways in wheat leaves IV. The interpretation of images of a fluorescent apoplastic tracer. Australian Journal of Plant Physiology. 1988;15:541–555. [Google Scholar]
- Canny MJ. Transfusion tissue of pine needles as a site of retrieval of solutes from the transpiration stream. New Phytologist. 1993;123:227–232. [Google Scholar]
- Chonan N, Kawahara H, Matsuda T. Ultrastructure of the small vascular bundles and transfer pathways for photosynthate in the leaves of rice plant. Japanese Journal of Crop Science. 1980;49:42–50. [Google Scholar]
- Chonan N, Kaneko M, Kawahara H, Matsuda T. Ultrastructure of the large vascular bundles in the leaves of rice plant. Japanese Journal of Crop Science. 1981;50:323–331. [Google Scholar]
- Chonan N, Kawahara H, Matsuda T. Ultrastructure of vascular bundles and fundamental parenchyma in relation to movement of photosynthate in leaf sheath of rice. Japanese Journal of Crop Science. 1984;53:435–444. [Google Scholar]
- Chonan N, Kawahara H, Matsuda T. Ultrastructure of the transverse veins in relation to phloem loading in the rice leaf. Japanese Journal of Crop Science. 1985;54:160–169. [Google Scholar]
- Evert RF, Botha CEJ, Mierzwa RJ. Free-space marker studies on the leaf of Zea mays L. Protoplasma. 1985;126:62–73. [Google Scholar]
- Evert RF, Eschrich W, Heyser W. Leaf structure in relation to solute transport and phloem loading in Zea mays L. Planta. 1978;138:279–294. doi: 10.1007/BF00386823. [DOI] [PubMed] [Google Scholar]
- Fritz E, Evert RF, Heyser W. Microautoradiographic studies of phloem loading in the leaf of Zea mays L. Planta. 1983;159:193–206. doi: 10.1007/BF00397525. [DOI] [PubMed] [Google Scholar]
- Heyser W, Evert RF, Fritz E, Eschrich W. Sucrose in the free space of translocation maize leaf bundles. Plant Physiolology. 1978;62:491–494. doi: 10.1104/pp.62.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsiliza B, Botha CEJ. Aphid (Sitobion yakini) investigation suggests thin-walled sieve tubes in barley (Hordeum vulgare) to be more functional than thick-walled sieve tubes. Physiologia Plantarum. 2002;115:137–143. doi: 10.1034/j.1399-3054.2002.1150116.x. [DOI] [PubMed] [Google Scholar]
- Opalka N, Brugidou C, Bonneau C, Nicole M, Beachy RN, Yeager M, Fauquet C. Movement of rice yellow mottle virus between xylem cells through pit membranes. Proceedings of the National Academy of Sciences, USA. 1998;95:3323–3328. doi: 10.1073/pnas.95.6.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer K, Wisniewski M. Development of the amorphous layer (protective layer) in xylem parenchyma of cv. Golden delicious apple cv. Loring. American Journal of Botany. 1989;76:1569–1582. [Google Scholar]
- Thomasson JR, Nelson ME, Zakrzewski RJ. A fossil grass (Gramineae: Chloridoideae) from the Miocene with Kranz anatomy. Science. 1986;233:876–878. doi: 10.1126/science.233.4766.876. [DOI] [PubMed] [Google Scholar]
- Van Bel AJE Xylem–phloem exchange via the rays: the undervalued route of transport. Journal of Experimental Botany. 1990;41:631–636. [Google Scholar]
- Van Bel AJE Strategies of phloem loading. Annual Review of Plant Physiology and Plant Molecular Biology. 1993;44:253–281. [Google Scholar]
- Wisniewski M, Davis G, Arora R. Effect of macerase, oxalic acid and EGTA on deep supercooling and pit membrane structure of xylem parenchyma of peach. Plant Physiology. 1991;96:1354–1359. doi: 10.1104/pp.96.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KM, Horobin RW, Oparka KJ. Phloem mobility of fluorescent xenobiotics in Arabidopsis in relation to their physicochemical properties. Journal of Experimental Botany. 1996;47:1779–1787. [Google Scholar]
- Wright KM, Oparka KJ. Uptake of Lucifer Yellow CH into plant-cell protoplasts: a quantitative assessment of fluid-phase endocytosis. Protoplasma. 1989;79:257–264. doi: 10.1007/BF00393697. [DOI] [PubMed] [Google Scholar]
- Zwieniecki MJ, Melcher PJ, Holbrook NM. Hydrogel control of xylem hydraulic resistance in plants. Science. 2001;291:1059–1062. doi: 10.1126/science.1057175. [DOI] [PubMed] [Google Scholar]