Abstract
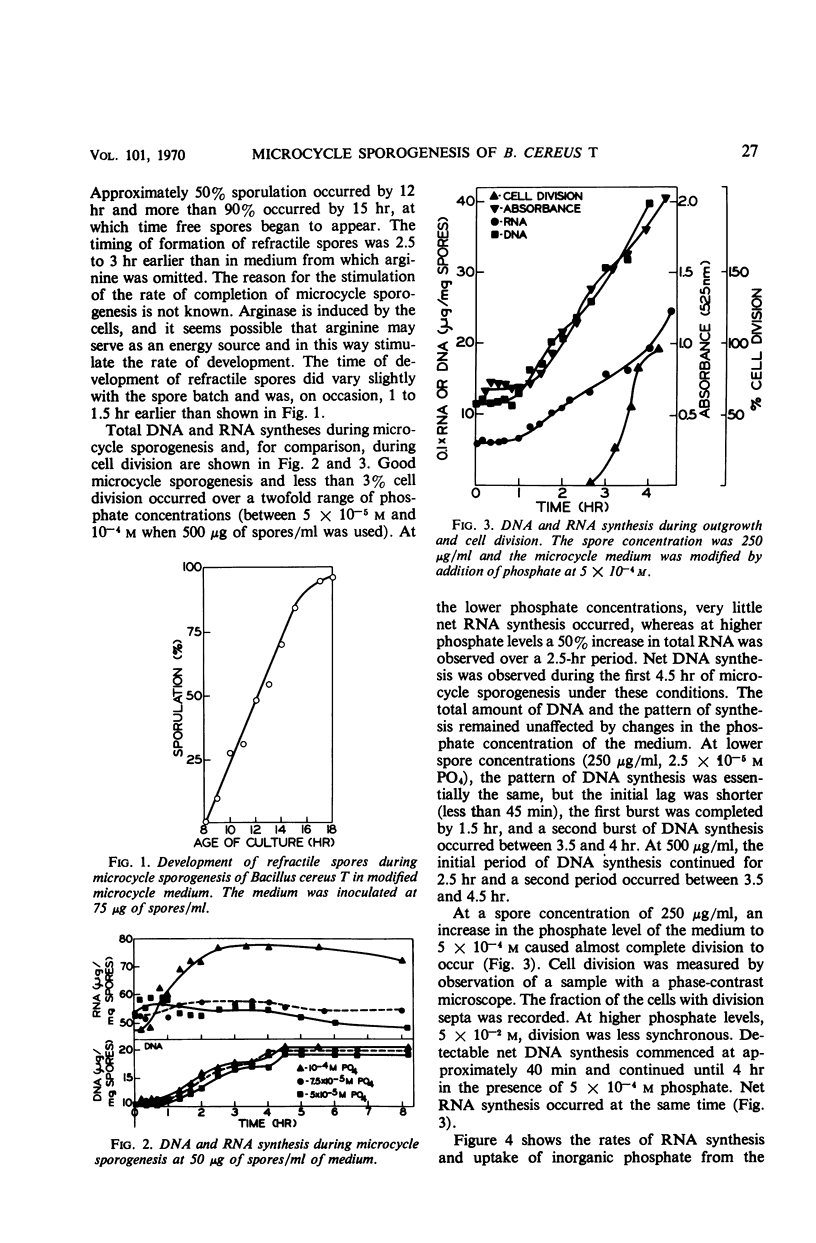
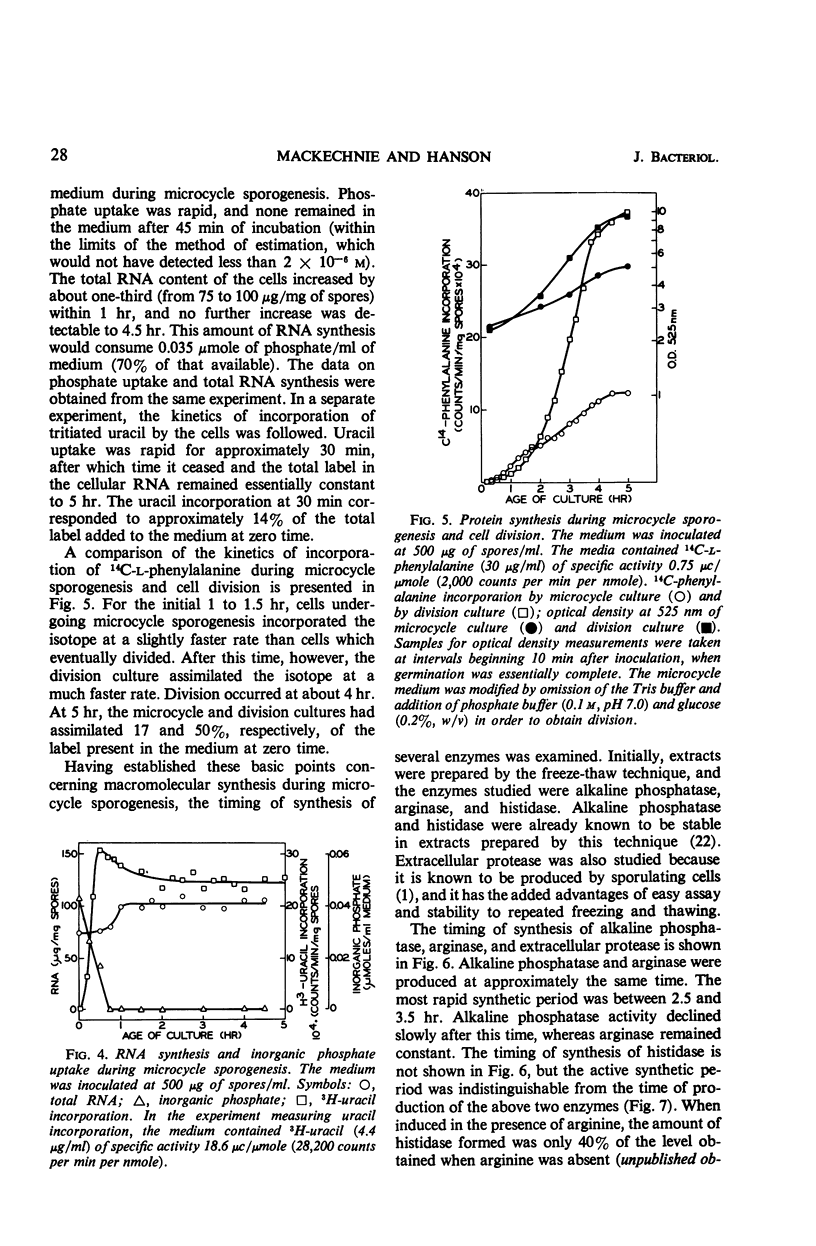
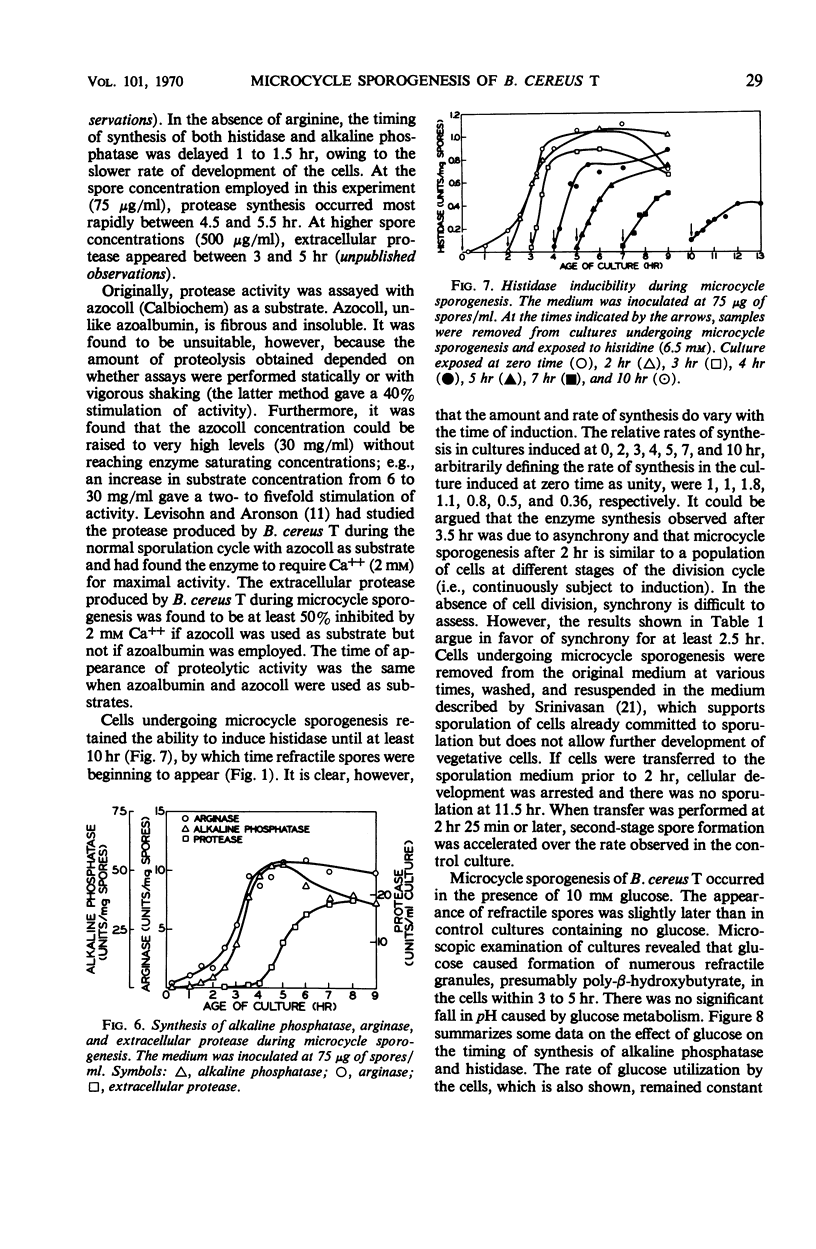
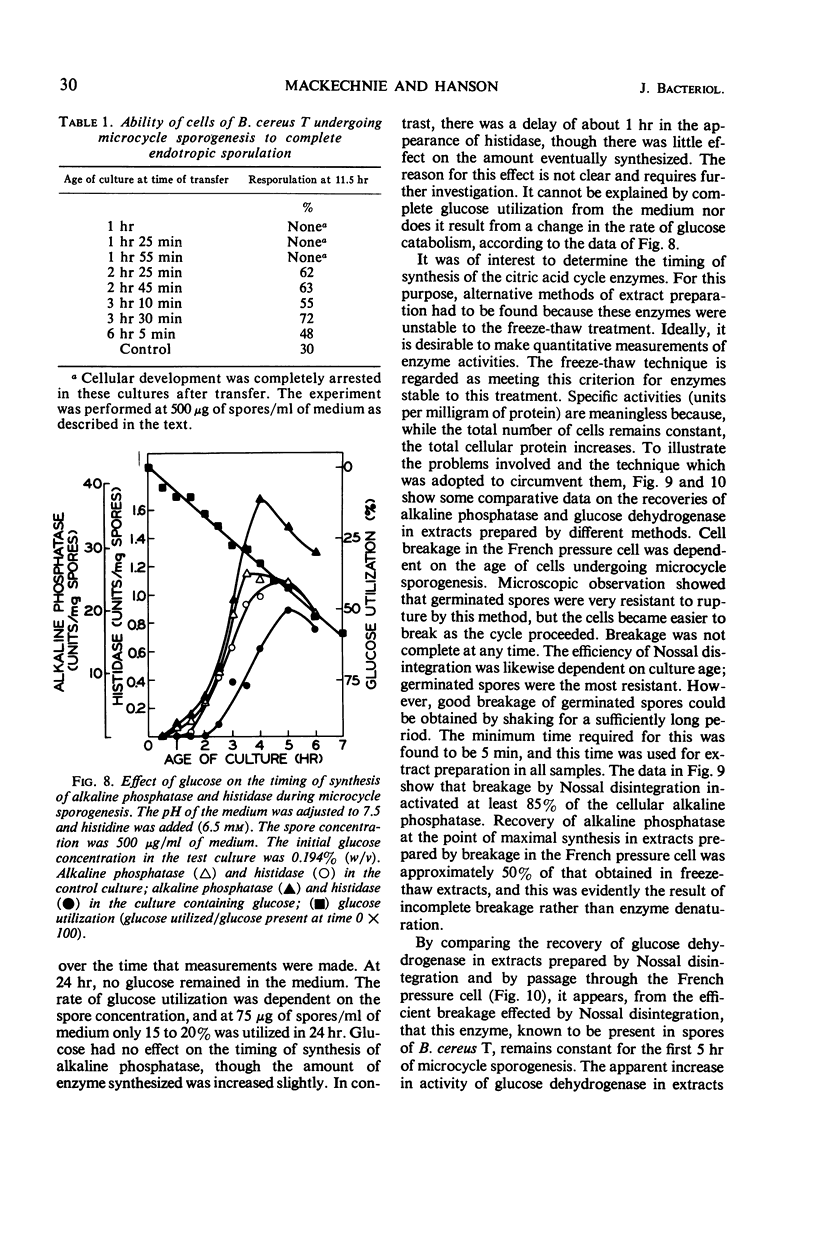
Microcycle sporogenesis induced in Bacillus cereus T by phosphate limitation occurs over a narrow range of phosphate to spore inoculum ratios. Sufficient phosphate is required to satisfy the demands for a twofold increase in deoxyribonucleic acid; net ribonucleic acid synthesis is not required. The total ribonucleic acid content of the culture was variable, and deoxyribonucleic acid synthesis was restricted to a twofold increase. Developmental changes during outgrowth occurred synchronously, whereas enzyme synthesis was periodic. The timing of the synthesis of tricarboxylic cycle enzymes, extracellular protease, arginase, histidase, and alkaline phosphatase was measured. Histidase could be induced after 2.5 hr throughout microcycle sporogenesis. Several other features of macromolecular synthesis during microcycle sporogenesis are described. Differences between this pattern and those observed during outgrowth leading to cell division are discussed. A technique for accurately estimating the levels and time of synthesis of incompletely extractable, labile enzymes is also presented.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balassa G. Quantitative regulation of RNA synthesis during sporulation of Bacillus subtilis. Biochem Biophys Res Commun. 1964 Mar 26;15(3):236–239. doi: 10.1016/0006-291x(64)90152-4. [DOI] [PubMed] [Google Scholar]
- GOLLAKOTA K. G., HALVORSON H. O. Biochemical changes occurring during sporulation of Bacillus cereus. Inhibition of sporulation by alpha-picolinic acid. J Bacteriol. 1960 Jan;79:1–8. doi: 10.1128/jb.79.1.1-8.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. Biochemistry of sporulation. I. Metabolism of acetate by vegetative and sporulating cells. J Bacteriol. 1963 Feb;85:451–460. doi: 10.1128/jb.85.2.451-460.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTWELL L. H., MAGASANIK B. THE MOLECULAR BASIS OF HISTIDASE INDUCTION IN BACILLUS SUBTILIS. J Mol Biol. 1963 Oct;7:401–420. doi: 10.1016/s0022-2836(63)80033-9. [DOI] [PubMed] [Google Scholar]
- Holmes P. K., Levinson H. S. Metabolic requirements for microcycle sporogenesis of Bacillus megaterium. J Bacteriol. 1967 Aug;94(2):434–440. doi: 10.1128/jb.94.2.434-440.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyem T., Rodenberg S., Douthit H. A., Halvorson H. O. Changes in the pattern of proteins synthesized during outgrowth and microcycle in Bacillus cereus T. Arch Biochem Biophys. 1968 Jun;125(3):964–974. doi: 10.1016/0003-9861(68)90535-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levisohn S., Aronson A. I. Regulation of extracellular protease production in Bacillus cereus. J Bacteriol. 1967 Mar;93(3):1023–1030. doi: 10.1128/jb.93.3.1023-1030.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKechnie I., Hanson R. S. Microcycle sporogenesis of Bacillus cereus in a chemically defined medium. J Bacteriol. 1968 Feb;95(2):355–359. doi: 10.1128/jb.95.2.355-359.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Ramaley R. F., Bernlohr R. W. Postlogarithmic phase metabolism of sporulating microorganisms. II. The occurrence and partial purification of an arginase. J Biol Chem. 1966 Feb 10;241(3):620–623. [PubMed] [Google Scholar]
- Rodenberg S., Steinberg W., Piper J., Nickerson K., Vary J., Epstein R., Halvorson H. O. Relationship between protein and ribonucleic acid synthesis during outgrowth of spores of Bacillus cereus. J Bacteriol. 1968 Aug;96(2):492–500. doi: 10.1128/jb.96.2.492-500.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg W., Halvorson H. O. Timing of enzyme synthesis during outgrowth of spores of Bacillus cereus. I. Ordered enzyme synthesis. J Bacteriol. 1968 Feb;95(2):469–478. doi: 10.1128/jb.95.2.469-478.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg W., Halvorson H. O. Timing of enzyme synthesis during outgrowth of spores of Bacillus cereus. II. Relationship between ordered enzyme synthesis and deoxyribonucleic acid replication. J Bacteriol. 1968 Feb;95(2):479–489. doi: 10.1128/jb.95.2.479-489.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- Vinter V., Slepecky R. A. Direct Transition of Outgrowing Bacterial Spores to New Sporangia Without Intermediate Cell Division. J Bacteriol. 1965 Sep;90(3):803–807. doi: 10.1128/jb.90.3.803-807.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]


