Abstract
Our current knowledge on sound detection in fishes is mainly based on data acquired under quiet laboratory conditions. However, it is important to relate auditory thresholds to background noise in order to determine the signal-detecting abilities of animals in the natural environment. We investigated the influence of two noise levels within the naturally occurring range on the auditory sensitivity of two hearing specialists (otophysines) and a hearing generalist. Audiograms of the goldfish Carassius auratus, the lined Raphael catfish Platydoras costatus and the pumpkinseed sunfish Lepomis gibbosus (hearing generalist) were determined between 200 and 4000 Hz (100–800 Hz for L. gibbosus) under laboratory conditions and under continuous white noise by recording auditory evoked potentials (AEPs). Baseline thresholds showed greatest hearing sensitivity around 500 Hz in goldfish and catfish and at 100 Hz in the sunfish. Continuous white noise of 110 dB RMS elevated the thresholds by 15–20 dB in C. auratus and by 4–22 dB in P. costatus. White noise of 130 dB RMS elevated overall hearing thresholds significantly in the otophysines by 23–44 dB. In the goldfish, threshold did not shift at 4 kHz. In contrast, auditory thresholds in the sunfish declined only at the higher noise level by 7–11 dB. Our data show that the AEP recording technique is suitable for studying masking in fishes, and that the occurrence and degree of the threshold shift (masking) depend on the hearing sensitivity of fishes, the frequency, and noise levels tested. The results indicate that acoustic communication and orientation of fishes, in particular of hearing specialists, are limited by noise regimes in their environment.
Keywords: auditory evoked potential, auditory sensitivity, hearing specializations, masking, teleosts
Introduction
The auditory system is particularly important for aquatic vertebrates when visual orientation is restricted. Sounds from different sources provide them with information relevant for survival, e.g., finding mates and prey or avoiding predators. The natural environment of fishes, especially that of marine fishes (Knudsen et al. 1948; Wenz 1962; Urick 1983; Myrberg 1990), but also freshwater habitats (Hawkins and Johnstone 1978; Rogers and Cox 1988; Lugli and Fine 2003), is characterized by a permanent background noise of abiotic (currents, rain, seismic events, coastal surf) and biotic (vocalizations of animals, photosynthesis) origin. In addition, the amount of man-made noise caused by ship and air traffic, hydroelectric power plants, or drilling is increasing. Thus noise is an omnipresent environmental constraint on the auditory system of fishes and ultimately determines the detectability of sounds relevant to their orientation toward prey, predators, and conspecifics, and to acoustic communication in their environment.
Most investigations on sound detection in fishes, however, were performed under quiet laboratory conditions, and their results may be ill-suited to information on the ability of fishes to detect signals in their natural environment. In terrestrial animals it is long known (e.g., Fletcher 1940) that the detection of one signal can be impaired by the presence of another (i.e., noise), a phenomenon termed masking. Several prior investigations have addressed this issue in fishes. Tavolga (1967) and Buerkle (1968, 1969) observed elevated auditory thresholds in the presence of increased background noise in the squirrelfish Holocentrus rufus, the grunt Haemulon sciurus, and the cod Gadus morhua. Studies conducted in the field on several other marine teleosts (Melanogrammusaeglefinus, Pollachiuspollachius, G. morhua, Molva molva; Chapman 1973; Chapman and Hawkins 1973) have confirmed that masking can occur even under relatively quiet sea conditions, suggesting that absolute sensitivity of the auditory system is less important than the ability to discriminate between relevant sound stimuli and background noise. All those experiments together pointed out the need to relate auditory thresholds to background noise in order to determine the signal-detecting abilities in the natural environment.
The main objectives of our study were to investigate (1) to which extent white noise at naturally occurring sound pressure levels (SPL) affect hearing thresholds, (2) to which degree different frequencies are masked, and (3) whether fishes with different hearing abilities are differently affected by similar noise levels. A further goal (4) was to evaluate the usefulness of the auditory evoked potential (AEP) method in studying masking in fishes by comparing our results to behaviorally obtained data (Fay 1974; Fay and Coombs 1983) in goldfish. This is a first step before addressing a wider range of issues related to the impacts of natural noise on the biology of fishes.
Our test animals were two hearing specialists, the cypriniform Carassius auratus (goldfish) and the sound-producing siluriform Platydoras costatus (lined Raphael catfish), and a vocal hearing generalist, the perciform Lepomis gibbosus (pumpkinseed sunfish). Noise is highly variable in the natural environment (Wenz 1962; Hawkins and Johnstone 1978; Urick 1983) and its biological relevance mostly unknown. Therefore and in order to compare our results to previous data, we applied white (Gaussian) noise with a relatively flat frequency spectrum as a masker.
Materials and methods
Animals
Test subjects were seven goldfish C. auratus (88–98 mm standard length, 22.3–28 g body weight) from a pond near Vienna, six lined Raphael catfish P. costatus (99–124 mm standard length; 22.9–38.8 g body mass), and seven pumpkinseed sunfish L. gibbosus (79–94 mm; 15.6–26.2 g). The latter two species were obtained from local pet suppliers. Goldfish were chosen because there exists a large amount of behavioral and neurophysiological data on diverse aspects of their hearing abilities including behavioral masking which are available from prior studies (Fay 1974; Fay and Coombs 1983). All animals were kept in planted aquaria whose bottoms were covered with sand, equipped with half flower pots as hiding places, filtered by external filters, and maintained at a 12L:12D cycle. The fishes were fed live Tubifex sp., chironomid larvae, or commercially prepared flake food (Tetramin®) daily. No submerged filters or air stones were used in order to reduce noise in the holding tanks. Background noise in the holding tanks ranged from 110 to 115 dB LLeq for the otophysines, and from 124 to 127 dB LLeq for L. gibbosus. All experiments were performed with the permission of the Austrian Commission on Experiments in Animals (GZ 68.210/50-Pr/4/2002).
Auditory evoked potential recordings
The AEP recording protocol used in this study followed that recently described in Wysocki and Ladich (2001, 2002, 2003). Therefore, only a brief summary of the basic technique is given here. During the experiments, the fishes were mildly immobilized with Flaxedil (gallamine triethiodide; Sigma). The dosage used was 0.90–1.9 μg g−1 for C. auratus, 1.3–3.3 μg g−1 for P. costatus, and 2.4–5.8 μg g−1 for L. gibbosus. This dosage allowed the fishes to retain slight opercular movements during the experiments but without significant myogenic noise to interfere with the recording. Test subjects were secured in a bowl-shaped plastic tub (diameter: 37 cm, water depth: 8 cm, 2 cm layer of fine sand) lined on the inside with acoustically absorbent material (air-filled packing wrap) in order to reduce resonances and reflections (for the illustration of the effect, see Fig. 1 in Wysocki and Ladich 2002). Fishes were positioned below the water surface (except for the contacting points of the electrodes, which were maximally 1 mm above the surface) in the center of the plastic tub.
A respiration pipette was inserted into the subject’s mouth. Respiration was achieved through a simple temperature-controlled (24 ± 1 °C), gravity-fed water circulation system. The AEPs were recorded by using silver wire electrodes (0.25 mm diameter) pressed firmly against the skin. The portion of the head above the water surface was covered by a small piece of Kimwipes tissue paper to keep it moist and to ensure proper contact during experiments. The recording electrode was placed in the midline of the skull over the region of the medulla and the reference electrode cranially between the nares. Shielded electrode leads were attached to the differential input of an a.c. preamplifier (Grass P-55, gain 100×, high-pass at 30 Hz, low-pass at 1 kHz). The plastic tub was positioned on an air table (TMC Micro-g 63–540) which rested on a vibration-isolated concrete plate. The entire setup was enclosed in a walk-in soundproof room, which was constructed as a Faraday cage (interior dimensions: 3.2 × 3.2 × 2.4 m).
Both sound stimuli presentation and AEP waveform recording were accomplished using a Tucker-Davis Technologies (Gainesville, FL, USA) modular rack-mount system (TDT System 3) controlled by a Pentium 4 PC containing a TDT digital processing board and running TDT BioSig RP Software.
Sound stimuli
Sound stimuli waveforms and masking noise were created using TDT SigGen RP software and fed through a power amplifier (Alesis RA 300). A dual-cone speaker (Tannoy System 600, frequency response 50 Hz–15 kHz ± 3 dB), mounted 1 m above test subjects in the air, was used to present the stimuli during testing.
Sound stimuli consisted of tone bursts which were presented at a repetition rate of 21 per second. Hearing thresholds were determined at frequencies of 200, 500, 1000, 2000, and 4000 Hz for C. auratus and P. costatus, and of 100, 200, 300, 500, and 800 Hz for L. gibbosus, presented in random order under normal laboratory conditions and in the presence of continuous masking noise. The duration of sound stimuli increased from two cycles at 100 and 200 Hz, up to eight cycles at 4 kHz. Rise and fall times were one cycle at 100 and 200 Hz, and two cycles at all the other frequencies. All bursts were gated using a Blackman window.
For each test condition, stimuli were presented at opposite polarities (180° phase shifted), and the corresponding AEPs averaged by the Bio-Sig RP software in order to eliminate stimulus artifacts. Sound-pressure levels of tone-burst stimuli were reduced in 4 dB steps until the AEP waveform was no longer apparent. The lowest SPL for which a repeatable AEP trace could be obtained, as determined by overlaying replicate traces, was considered the threshold (Kenyon et al. 1998).
A hydrophone (Brüel & Kjaer 8101, frequency range: 1 Hz–80 kHz ± 2 dB; voltage sensitivity: −184 re 1 V/μPa) was placed close to the right side of the animals (2 cm apart) in order to determine absolute SPLs underwater in close vicinity of the subjects. Control measurements showed that, in accordance with theoretical expectations (due to increasing distance from the loudspeaker), SPLs decreased with increasing distance from the center of the tub as well as with increasing depth. Our sound-pressure-sensitive hydrophone responded exactly to any attenuation in SPL generated by the BioSig software and played back via the air loudspeaker. A limitation always present in auditory experiments on fishes is the fact that the three-dimensional kinetic component (particle motion) of sounds cannot be measured exactly because of a lack of appropriate instruments, and therefore any description of the sound field will be incomplete. Results from prior studies and control experiments indicate that the use of an air speaker minimizes the classical near field effect. Enger (1966) showed that sound pressure thresholds depend highly on the distance to an underwater speaker because it creates a near-field effect in its vicinity leading to unproportional changes of sound pressure and particle motion. Sound pressure thresholds determined from stimulation with an air loudspeaker at 15 cm distance were similar to those determined with an underwater speaker at 2 m distance. We performed control experiments in our experimental tank to test whether pressure and particle motion change in correlation with each other, all the while changing the distance between the speaker and the test fishes. Sound pressure levels at hearing threshold were calibrated at every frequency and distance of the loudspeaker (1 vs. 0.5 m). The SPL of a given sound stimulus in the test tank besides the fish increased with decreasing distance of the loudspeaker by up to 11 dB. In contrast, the hearing thresholds of the sunfish to stimuli of 100, 200, 300, and 500 Hz measured in dB SPL showed no significant change at any frequency (paired t-tests, n.s. at all frequencies tested) confirming that the particle motion component did not increase unproportionally and thus the validity of our results.
Sound pressure is the adequate measure of the degree of auditory stimulation in pressure-sensitive fishes such as otophysines (Fay and Popper 1974) in any acoustic field. For technical and comparative reasons, the hearing thresholds of sunfish are also given in SPLs, although hearing generalists detect particle motion of sounds. This seemed to be adequate because our study emphasized the effects of the same defined background noise (noise spectra are always given in pressure units) on signal detection in different species using the same experimental setup and on relative threshold shifts within a species rather than absolute thresholds. This is valid as long as the displacement field is proportional to the pressure field, because in masking studies the ratio of the tone level to the noise level at nearby frequencies is most important. However, it should always be kept in mind that those hearing thresholds should not be regarded as absolute values because the exact proportional factor between the two sound parameters remains unknown.
Masking noise
Audiograms were measured under normal laboratory conditions and in the presence of continuous white noise at two different levels. The noise was created by the SigGen RP software, sent to a 30-band equalizer (Alesis MEQ 230) to obtain the flattest possible noise spectrum (see Fig. 1 for spectra) and fed to the second channel of an SM5 signal mixer (the tone burst signals were fed to the first channel of the signal mixer). Both signals were then fed via the Alesis RA 300 amplifier to the dual-cone speaker.
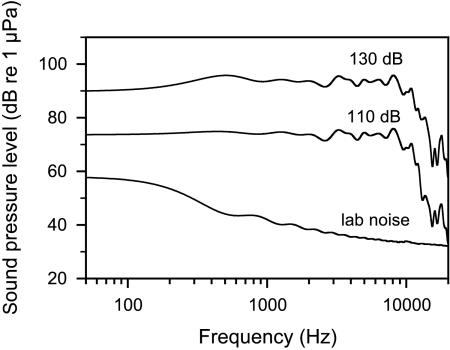
Fig. 1.
Cepstrum-smoothed (coefficients: 64) spectra of mean laboratory noise (lower line), white noise of 110 dB LLeq (mid line) and of 130 dB LLeq (upper line). Cepstrum-smoothed spectra are plotted for better representation. Cepstrum smoothing is a mathematical calculation (Noll 1967) for the representation of the mean energy content of fluctuating sound spectra.
The sound pressure levels of the masking noise were measured at the position of the fish using a Brüel & Kjaer 2238 Mediator, Brüel & Kjaer 2804 power supply, and a Brüel & Kjaer 8101 hydrophone determining the L-weighted (5 Hz to 20 kHz) equivalent continuous SPL (LLeq) averaged over 1 min measuring time. The Leq is a measure of the averaged energy in a varying sound level and commonly used to assess environmental noise (ISO 1996). The system was calibrated using a Brüel & Kjaer 4229 calibrator.
The LLeqs of the noise masker were 110 and 130 dB re 1 μPa. These sound levels were chosen because they cover the range of levels encountered in the natural environment, e.g., in Alpine foothill rivers and the Danube river (L.E. Wysocki, S. Amoser, F. Ladich, personal observation) and in fish-keeping facilities (Bart et al. 2001). In addition, background noise levels in the experimental test tank (normal laboratory conditions) were measured on different days at the position where fishes were tested. After each SPL measurement, the background noise and the white masking noise were recorded on a DAT recorder (Sony TCD 100) and then analyzed using S_Tools (STX 2.17), the Integrated Workstation for Acoustics, Speech, and Signal Processing developed by the Research Laboratory of Acoustics at the Austrian Academy of Sciences. Sound spectra of 1 min recordings were calculated by an FFT analysis using a filter bandwidth of 1 Hz. These spectra were then exported and the relative spectral values were transformed to linear values by using the equation:
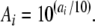 |
1 |
Ai is the linear spectral amplitude value and ai is the logarithmic spectral amplitude value.
From these values, the mean relative RMS was calculated by the equation:
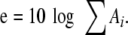 |
2 |
e is the mean RMS value calculated from the spectral amplitudes.
The mean relative RMS was then equaled to the absolute SPL measured with the mediator and the relative spectral levels were recalculated into absolute spectral levels. For laboratory ambient noise spectra, the absolute spectra of each measurement performed on different days were averaged in order to obtain a mean ambient noise spectrum (Fig. 1). Laboratory noise averaged 82.5 ± 0.5 dB LLeq.
Data analysis
Audiograms of the different experimental groups were compared by two-factor analysis of variance (ANOVA) using a general linear model where one factor was masking noise/noise condition and the other was frequency. The noise factor alone should indicate overall differences between masking conditions, and in combination with the frequency factor if different tendencies exist at different frequencies of the audiograms. To determine the frequency at which thresholds differ, paired t-tests were calculated at each frequency. Parametric statistical tests were applied because the data showed normal distribution and homogeneity of variances. All statistical tests were run using SPSS version 10.0.
Results
Effects of background noise on hearing thresholds
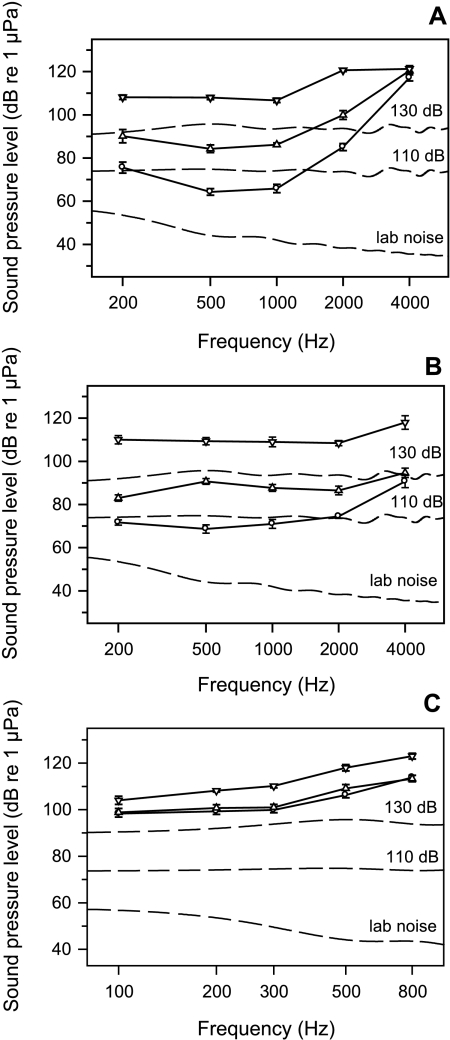
Baseline audiograms as measured under normal laboratory background noise of goldfish and catfish showed greatest hearing sensitivity at about 500 Hz (64.3 ± 1.8 dB SE for C. auratus and 68.7 ± 2.1 dB SE for P. costatus; Fig. 2; see also Ladich 1999).
Fig. 2.
Audiograms (solid lines) and appropriate cepstrum-smoothed noise spectra (dashed lines) of A. Carassius auratus, B. Platydoras costatus and C. Lepomis gibbosus. –○– Hearing thresholds obtained under normal laboratory conditions, –▵– hearing thresholds under masking noise of 110 dB, –▿– hearing thresholds under masking noise of 130 dB.
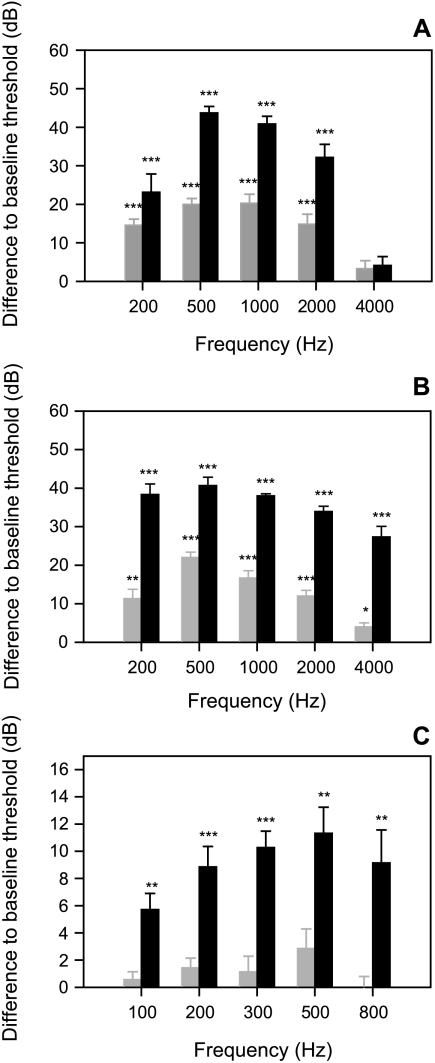
At a masking noise level of 110 dB LLeq, the mean hearing thresholds (average of all individuals at a particular frequency) of C. auratus increased by up to 20 dB and by up to 44 dB at a noise level of 130 dB (Fig. 2A). The amount of threshold shift differed between frequencies, being more pronounced in the most sensitive hearing range (500 and 1 kHz). At 130 dB, the whole audiogram became relatively flat. Paired t-tests showed significant differences between baseline and masked thresholds for both noise levels at all frequencies except 4 kHz (Fig. 3A).
Fig. 3.
Differences of thresholds determined under white noise of 110 dB (grey bars) and 130 dB (black bars) to hearing thresholds under normal laboratory conditions of A. Carassius auratus, B. Platydoras costatus and C. Lepomis gibbosus. Asterisks indicate levels of significance (paired t-test; *p ≤ 0.05; **p ≤ 0.01, ***p ≤ 0.001).
In the catfish P. costatus, the mean hearing thresholds (average of all individuals at a particular frequency) increased by up to 22 dB (500 Hz) at a noise level of 110 dB and by up to 41 dB (500 Hz) at 130 dB (Fig. 2B). Hearing sensitivity at a masking noise of 110 dB decreased especially in the midfrequency range (500 Hz). The masking effect was much smaller (only 4 ± 1.0 dB) but statistically significant (p = 0.012) at the highest frequency (4 kHz) tested. At 130 dB, hearing thresholds increased in addition. Similar to C. auratus, hearing thresholds at this noise level were above 100 dB at all frequencies. Paired t-tests showed significant differences between baseline and masked thresholds for both noise levels at all frequencies (Fig. 3B).
The sunfish L. gibbosus had a lower auditory sensitivity compared to both otophysines with the maximum sensitivity found at 100 Hz (98.3 ± 1.5 dB SE; Fig. 2C). White noise of 110 dB did not affect the auditory sensitivity. Paired t-tests showed no significant change in hearing threshold at any frequency. When animals were exposed to the higher noise level (130 dB), thresholds shifted up to 11 dB (500 Hz) compared to baseline thresholds (Fig. 3C).
Threshold-to-noise ratios
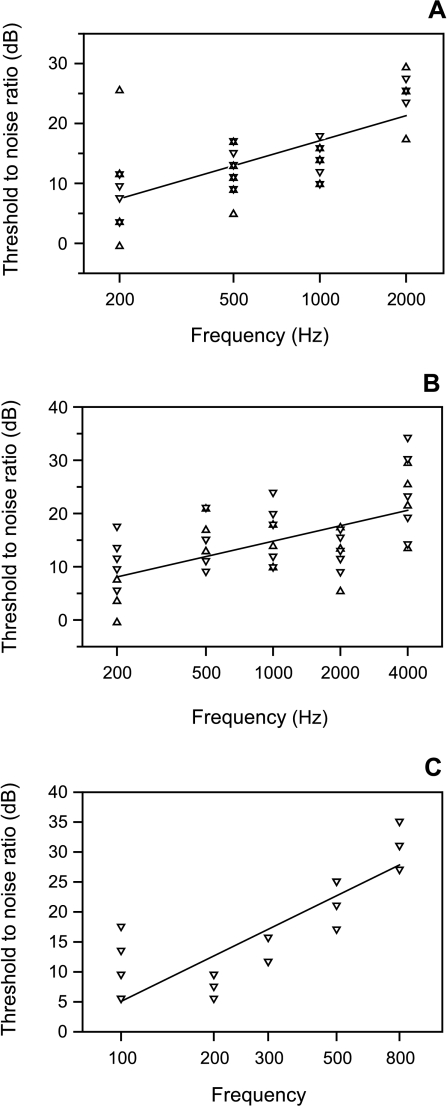
Threshold-to-noise (T/N) ratios were calculated by subtracting the spectrum level of noise (in a 1 Hz band) from the SPL at hearing threshold at this particular frequency for all masked thresholds of the three species. In C.auratus and P.costatus, threshold-to-noise ratios for the 110- and 130 dB masker were pooled, because statistical analysis (paired t-test at each frequency) showed no significant differences. The mean T/N ratios (±SE) increased with increasing frequency from 9.7 ± 1.6 to 25 ± 1.1 dB in C.auratus, from 7 ± 1.6 to 23.7 ± 1.9 dB in P.costatus, and from 9.6 ± 1.8 to 31.1 ± 1.2 dB in L.gibbosus.
The ratios were significantly correlated to the frequency tested (as calculated by first-order linear functions; Fig. 4A–C). All correlations were positive, indicating that the ratios increased with frequency.
Fig. 4.
Threshold-to-noise ratios for masked hearing thresholds of A. Carassius auratus, B. Platydoras costatus and C. Lepomis gibbosus. Data for the 110 dB (–▵–) and 130 dB (–▿–) noise are pooled in A and B, see explanation in text. A. T/N ratio = sound frequency × 0.0085 + 6.78, r = 0.80, p < 0.001. B. T/N ratio = sound frequency × 0.0034 + 9.47, r = 0.64, p < 0.001. C. T/N ratio = sound frequency × 0.034 + 4.05, r = 0.93, p < 0.001.
Discussion
Differential effects of background noise on hearing sensitivity
Per definition, masking occurs when the detection of one signal is impaired by another. This phenomenon is often neglected in fish audiometry. The large variability of goldfish and cod audiograms can most likely be explained by different background noise levels during experiments (Hawkins 1981). The AEP approach being presented here for studying masking in fishes enables us to investigate this phenomenon in a large number of species within a short period of time. This noninvasive approach provides a tool for a rapid evaluation of the hearing abilities of different species under similar or different conditions without lengthy or species-specific training, without harming the animals, and thus without limitations on repeated testing of the subjects—and is therefore particularly suitable for species comparisons.
The occurrence and degree of masking in the present study depended on the overall hearing sensitivity of the fish relative to the noise level. Continuous white noise of 110 and 130 dB LLeq significantly influenced auditory thresholds in both otophysine species, whereas in L. gibbosus the 110 dB noise did not shift hearing thresholds; only the 130 dB noise level evoked a smaller (as compared to the otophysines) loss in auditory sensitivity. Hearing generalists such as sunfish are generally less sensitive to sounds than hearing specialists (i.e., otophysines; Hawkins and Myrberg 1983; Popper and Fay 1993; Ladich and Popper, 2004). In our experiments, the spectrum level of the lower noise lies at least 25 dB below the hearing thresholds so that the noise has no masking effect. This is also true at the highest frequency for the goldfish.
The masking effect of a given noise level was maximal within the most sensitive hearing range of the otophysines (between 500 and 1000 Hz), whereas frequencies at the upper and lower ends of the audiograms were less affected or unaffected by masking noise. In C. auratus, no significant change in hearing threshold at either noise level was found at 4 kHz, where goldfish showed lowest hearing sensitivity under normal laboratory conditions and which is close to their upper hearing limit (Popper 1971; Kenyon et al. 1998). Similar findings were reported by Buerkle (1968) and Chapman and Hawkins (1973) on the cod G. morhua. In contrast, all thresholds in P. costatus were masked by the 110- and 130-dB noise. Compared to the goldfish, catfish are more sensitive in the high frequency range (Ladich 1999; Amoser and Ladich 2003; Ladich and Bass 2003). Hence interspecific variance in baseline auditory sensitivity accounts for variance in the degree of masking.
The auditory thresholds of the otophysines increased linearly with the background noise level within the best hearing range: A 20 dB increase of white noise (110 vs. 130 dB) increased the masked hearing thresholds by about 20 dB in the goldfish (except at 4 kHz) and in the catfish at frequencies ranging from 500 Hz to 4 kHz. Our AEP results agree with other studies using various techniques and maskers. Almost linear relationships between noise levels and masked hearing thresholds (behavioral or single unit) have been found in several species such as in H. rufus (Tavolga 1967), G. morhua (Buerkle 1968; Enger 1973; Chapman and Hawkins 1973), goldfish (Fay 1974), the spotted shad Clupanodon punctatus, and Asian greenling Pleurogrammus azonus (Sorokin 1989). This correlation is mainly found in the frequency range in which an animal is most sensitive to sounds, and it is not valid at the upper and lower ends of its hearing range.
The data on fishes generally agree with the psychophysically obtained description of masking phenomena in mammals despite the differences in the morphology and physiology of inner ears and auditory pathways. This includes the fact that masked hearing thresholds for tones are less dependent on the frequency of the tone than in unmasked conditions, and that for each dB increase of the masker, the signal must be increased by the same amount to maintain constant detection. This may be based on a sort of level discrimination ability (Yost 2000).
The present results are in good accordance with behavioral data of prior studies on different fish species with regard to masking phenomena despite differences in methodological approaches. This demonstrates the usefulness of the AEP approach for investigating masking and effects of noise on fishes. In addition, they provide, by direct comparison, evidence for pronounced species differences in the effects of noise; this is an important factor which has to be considered when addressing questions related to communicative and environmental issues.
Threshold-to-noise ratios and implications for their biological significance
A key factor for understanding the influence of environmental noise on signal detection and acoustic communication in fishes is the threshold-to-noise (T/N) ratio, which quantifies auditory masking. It is defined as the difference (in dB) between the masked hearing threshold and the spectrum level of the masking noise (Chapman and Hawkins 1973).
For G. morhua, T/N ratios of masked hearing thresholds are reported to range from 18 to 36 dB by Buerkle (1968), and from 16 to 21 dB by Hawkins and Chapman (1975). The ratios for cod at comparable frequencies differed by about 5 to 15 dB. Fay (1974) and Fay and Coombs (1983), using a masking noise with a flat spectrum in the frequency regions of the signals used, obtained T/N ratios for goldfish ranging from 14 to 25 dB. At similar frequencies, this differs from our results in the same species (goldfish were tested in order to evaluate the method) by 6–11 dB. Possible explanations for within-species differences found in cod and goldfish are methodological differences concerning the acoustic stimuli (duration and onset time of the signals, bandwidth, and spectrum shape of the masking noise) as well as (genetic) differences between the fish strains. Fay and Coombs (1983) have shown that phase-locking synchronization thresholds of afferent neurons were well below signal-to-noise (S/N) levels at which spike rate increments were observed. Comparing those neurophysiological results to psychophysically obtained T/Ns, the authors concluded that spike rate increments probably provide the information used in behavioral detection decisions; alternatively, behavioral detection could be based upon the attainment of a certain degree of synchronization between the stimulus waveform and the evoked spike trains. This observation that neural synchronization starts at S/N ratios below behavioral thresholds can additionally explain the relatively low T/Ns measured in the present study because AEPs reflect synchronous activation, primarily of onset-type neurons within the auditory system. The present results, however, closely correspond to those obtained in psychophysical investigations on goldfish by Fay (1974): (1) in the increase of the T/N ratio with frequency and (2) that within the noise ranges tested, the ratios did not significantly differ at various noise levels within each frequency. Again, this supports the usefulness of the AEP approach in investigating masking phenomena in fishes, particularly for between-species comparisons and repeated measurements under different conditions.
Another aspect connected to the T/N ratios is the concept of the critical band. The results of several bandshift experiments on mammals and fishes (Fletcher 1940; Buerkle 1969; Tavolga 1974; Hawkins and Chapman 1975; Fay et al. 1978) have shown that the energy of the masker contributes to the masking effect only within a certain frequency band (generally termed critical band; Fletcher 1940). The mammalian auditory system is therefore typically viewed as segregating acoustic signals into their constituent frequencies in a manner analogous to the operation of overlapping bandpass filters. According to Fletcher (1940), the critical bandwidth of the auditory filter can be estimated indirectly under the assumption that the power of a signal at the masked hearing threshold is equal to the total noise power within the critical band, a method referred to as the critical ratio equal power (CR/EqP) method (Richardson et al. 1995). While such indirect estimates of the critical bandwidth are consistently smaller than direct measurements (e.g., by the factor 2.5 in humans), the trend to increasing bandwidths with increasing frequency (reflected in lower T/Ns at lower frequencies) is preserved (Long 1994; Yost 2000; Southall et al. 2003). In mammals, such characteristics of the auditory filters have been attributed to nonlinear properties of cochlea transduction (Long 1994; Southall et al. 2003). However, the above-mentioned phenomena (increasing T/N ratio with frequency, independence of T/N ratios of masking level over a wide range of levels) also seem to be a common trend in fishes. Given the absence of a cochlea in fishes, their comparatively low T/N ratios have been speculated to rely on their particularly well developed temporal analyzing power (Fay 1974), enabling them to efficiently extract signals from noise.
Sorokin (1989), investigating three species of marine fish, found T/N ratios in spotted shad C. punctatus and Asian greenlings P. azonus ranging from 16 to 21 dB at frequencies below 125 Hz. Within a certain variance of ambient noise, these ratios remained constant within a given frequency. When artificial, high-level noise was applied as a masker, the ratios declined by 2–5 dB in both species. Sorokin interpreted this difference in ratios between low- and high-level noise as an adaptation to noisy marine environments. T/N ratios of odontocetes and pinnipeds are also relatively low compared to most terrestrial mammals (Richardson et al. 1995; Southall et al. 2000). This has led to speculations that acoustic signal production and reception in noisy marine environments has promoted selection for the enhanced ability to detect signals in noise (Schusterman et al. 2000): A low T/N ratio at a particular frequency indicates relatively efficient extraction of signals from noise (a smaller bandwidth of the noise is effective in masking) compared to higher ratios and a higher frequency-resolving capacity of the auditory system (Fay 1974; Southall et al. 2003).
Indeed, masking is likely to occur in most marine environments, especially in coastal waters (Fay et al. 1978; Popper and Clarke 1979; Ladich and Popper 2004), suggesting that background noise (including that of anthropogenic origin) is an important environmental constraint for signal detection and for acoustic orientation and communication in the natural habitats of fishes. Considerable background noise also occurs in certain freshwater habitats, e.g., rivers (Lugli and Fine 2003), giving rise to speculation that ambient sounds in the environment have influenced the evolution of sound detection or source segregation in fishes (Schellart and Popper 1992).
Due to differences in overall auditory abilities, however, some species seem inherently less limited by naturally occurring noise levels than others (Hawkins and Myrberg 1983). Thus field studies show that cods are substantially affected by altered ambient noise in their natural environment (Chapman and Hawkins 1973; Hawkins and Chapman 1975), whereas Atlantic salmon Salmo salar were only masked by sea noise at levels substantially above the ambient levels in one of its habitats (a Scottish loch; Hawkins and Johnstone 1978). The present study has demonstrated that sunfish are substantially less affected by the same amount of background noise (because of their lower hearing sensitivity) than two otophysines, although there is only a small difference in the T/N ratio of these three species.
The concerns raised about the ever-increasing amount of anthropogenic noise (Andrew et al. 2002) and its impact on fishes call for a more detailed knowledge about noise effects on hearing in fishes, especially with regard to species differences. Threshold-to-noise ratios, combined with other data on physiological and filtering mechanisms underlying signal detection in noise, will help us estimate the impact zones of anthropogenic noise sources in fishes.
Acknowledgments
We are grateful to Andrzej Szpetkowski for his help in setting up the TDT system 3; to Anton Noll for providing formulas for sound analysis and noise spectra calculations; and to Michael Stachowitsch for professional scientific English proofreading. This study was supported by the Austrian Science Fund (FWF grant No. 15873 to F.L.).
References
- Amoser S, Ladich F. Diversity in noise-induced temporary hearing loss in otophysine fishes. J. Acoust. Soc. Am. 2003;113:2170–2179. doi: 10.1121/1.1557212. [DOI] [PubMed] [Google Scholar]
- Andrew RK, Howe BM, Mercer JA, Dzieciuch MA. Ocean ambient sound: comparing the 1960s with the 1990s for a receiver off the California coast. Acoust. Res. Lett. Online. 2002;3:65–70. [Google Scholar]
- Bart AN, Clark J, Young J, Zohar Y. Underwater ambient noise measurements in aquaculture systems: a survey. Aquac. Eng. 2001;25:99–110. [Google Scholar]
- Buerkle U. Relation of pure tone thresholds to background noise level in the Atlantic cod (Gadus morhua) J. Fish. Res. Board Can. 1968;25:1155–1160. [Google Scholar]
- Buerkle U. Auditory masking and the critical band in Atlantic cod (Gadus morhua) J. Fish. Res. Board Can. 1969;26:1113–1119. [Google Scholar]
- Chapman CJ. Field studies of hearing in teleost fish. Helgol. Wiss. Meeresunters. 1973;24:371–390. [Google Scholar]
- Chapman CJ, Hawkins AD. A field study of hearing in the cod, Gadus morhua. L. J. Comp. Physiol. 1973;85:147–167. [Google Scholar]
- Enger PS. Acoustic threshold in goldfish and its relation to the sound source distance. Comp. Biochem. Physiol. 1966;18:859–868. doi: 10.1016/0010-406x(66)90218-0. [DOI] [PubMed] [Google Scholar]
- Enger PS. Masking of auditory responses in the medulla oblongata of goldfish. J. Exp. Biol. 1973;59:415–424. doi: 10.1242/jeb.59.2.415. [DOI] [PubMed] [Google Scholar]
- Fay RR. Masking of tones by noise for the goldfish (Carassius auratus) J. Comp. Psychol. 1974;87:708–716. doi: 10.1037/h0037002. [DOI] [PubMed] [Google Scholar]
- Fay RR, Coombs SL. Neural mechanisms in sound detection and temporal summation. Hear. Res. 1983;10:69–92. doi: 10.1016/0378-5955(83)90018-7. [DOI] [PubMed] [Google Scholar]
- Fay RR, Popper AN. Acoustic stimulation of the ear of the goldfish, (Carassius auratus) J. Exp. Biol. 1974;61:243–260. doi: 10.1242/jeb.61.1.243. [DOI] [PubMed] [Google Scholar]
- Fay RR, Ahroon WA, Orawski AA. Auditory masking patterns in the goldfish (Carassius auratus): psychophysical tuning curves. J. Exp. Biol. 1978;74:83–100. doi: 10.1242/jeb.74.1.83. [DOI] [PubMed] [Google Scholar]
- Fletcher H. Auditory patterns. Rev. Mod. Phys. 1940;12:47–65. [Google Scholar]
- Hawkins AD. The hearing abilities of fish. In: Pitcher T, editor. Behavior of Teleost Fishes. London: Chapman & Hall; 1981. pp. 129–169. [Google Scholar]
- Hawkins AD, Chapman CJ. Masked auditory thresholds in the cod, Gadus morhua. L. J. Comp. Physiol. A. 1975;103:209–226. [Google Scholar]
- Hawkins AD, Johnstone ADF. The hearing of the Atlantic salmon, Salmo salar. J. Fish Biol. 1978;13:655–673. [Google Scholar]
- Hawkins AD, Myrberg AA. Hearing and sound communication under water. In: Lewis B, editor. Bioacoustics, A Comparative Approach. London: Academic Press; 1983. pp. 373–387. [Google Scholar]
- ISO 1996. Description, measurement and assessment of environmental noise. International Organization for Standardization, 2003.
- Kenyon TN, Ladich F, Yan HY. A comparative study of hearing ability in fishes: The auditory brainstem response approach. J. Comp. Physiol. A. 1998;182:307–318. doi: 10.1007/s003590050181. [DOI] [PubMed] [Google Scholar]
- Knudsen VO, Alford RS, Emling JW. Underwater ambient noise. J. Mar. Res. 1948;3:410–429. [Google Scholar]
- Ladich F. Did auditory sensitivity and vocalization evolve independently in otophysan fishes? Brain Behav. Evol. 1999;53:288–304. doi: 10.1159/000006600. [DOI] [PubMed] [Google Scholar]
- Ladich F, Bass AH. Audition. In: Arratia G, Kapoor BG, Chardon M, Diogo R (eds) Catfishes, vol. 2. Science Publishers Inc., Enfield, NH, pp. 701–730, 2003.
- Ladich F, Popper AN. Parallel evolution of fish hearing organs. In: Manley GA, Popper AN, Fay RR, editors. Evolution of the Vertebrate Auditory System. New York: Springer Verlag; 2004. pp. 95–127. [Google Scholar]
- Long GR. Psychoacoustics. In: Fay RR, Popper AN, editors. Comparative Hearing: Mammals. New York: Springer Verlag; 1994. pp. 18–56. [Google Scholar]
- Lugli M, Fine ML. Acoustic communication in two freshwater gobies: Ambient noise and short-range propagation in shallow streams. J. Acoust. Soc. Am. 2003;114:512–521. doi: 10.1121/1.1577561. [DOI] [PubMed] [Google Scholar]
- Myrberg AA. The effects of man-made noise on the behavior of marine mammals. Environ. Int. 1990;16:575–586. [Google Scholar]
- Noll AM. Cepstrum pitch determination. J. Acoust. Soc. Am. 1967;41:293–309. doi: 10.1121/1.1910339. [DOI] [PubMed] [Google Scholar]
- Popper AN. The effects of size on auditory capacities of the goldfish. J. Aud. Res. 1971;11:239–247. [Google Scholar]
- Popper AN, Clarke NL. Non-simultaneous auditory masking in the goldfish, Carassius auratus. J. Exp. Biol. 1979;83:145–158. doi: 10.1242/jeb.83.1.145. [DOI] [PubMed] [Google Scholar]
- Popper AN, Fay RR. Sound detection and processing by fish: Critical review and major research questions. Brain. Behav. Evol. 1993;41:14–38. doi: 10.1159/000113821. [DOI] [PubMed] [Google Scholar]
- Richardson JW, Greene CR, Malme CI, Thomson DH. Marine Mammals and Noise. New York: Academic Press; 1995. [Google Scholar]
- Rogers PH, Cox M. Underwater sound as a biological stimulus. In: Atema J, Fay RR, Popper AN, Tavolga WN, editors. Sensory Biology of Aquatic Animals. New York: Springer; 1988. pp. 131–149. [Google Scholar]
- Schellart NAM, Popper AN. Functional aspects of the evolution of the auditory system of actinopterygian fish. In: Webster DE, Fay RR, Popper AN, editors. The Evolutionary Biology of Hearing. New York: Springer; 1992. pp. 295–322. [Google Scholar]
- Schusterman RJ, Kastak D, Levenson DH, Reichmuth CJ, Southall BL. Why pinnipeds don’t echolocate. J. Acoust. Soc. Am. 2000;107:2256–2264. doi: 10.1121/1.428506. [DOI] [PubMed] [Google Scholar]
- Sorokin MA. Detection of acoustic signals in noise by fish. Biol. Nauki. 1989;6:35–40. [Google Scholar]
- Southall BL, Schusterman RJ, Kastak D. Masking in three pinnipeds: underwater, low-frequency critical ratios. J. Acoust. Soc. Am. 2000;108:1322–1326. doi: 10.1121/1.1288409. [DOI] [PubMed] [Google Scholar]
- Southall BL, Schusterman RJ, Kastak D. Auditory masking in three pinnipeds: Aerial critical ratios and direct critical bandwidth measurements. J. Acoust. Soc. Am. 2003;114:1660–1666. doi: 10.1121/1.1587733. [DOI] [PubMed] [Google Scholar]
- Tavolga WN. Masked auditory thresholds in teleost fishes. In: Tavolga WN, editor. Marine Bio-Acoustics. Oxford: Pergamon Press; 1967. pp. 233–243. [Google Scholar]
- Tavolga WN. Signal/noise ratio and the critical band in fishes. J. Acoust. Soc. Am. 1974;55:1323–1333. doi: 10.1121/1.1914704. [DOI] [PubMed] [Google Scholar]
- Urick RJ. Principles of Underwater Sound. Los Altos, CA: Peninsula Publishing; 1983. [Google Scholar]
- Wenz GM. Acoustic ambient noise in the ocean: spectra and sources. J. Acoust. Soc. Am. 1962;34:1936–1956. [Google Scholar]
- Wysocki LE, Ladich F. The ontogenetic development of auditory sensitivity, vocalization and acoustic communication in the labyrinth fish Trichopsis vittata. J. Comp. Physiol. A. 2001;187:177–187. doi: 10.1007/s003590100186. [DOI] [PubMed] [Google Scholar]
- Wysocki LE, Ladich F. Can fishes resolve temporal characteristics of sounds? New insights using auditory brainstem responses. Hear. Res. 2002;169:36–46. doi: 10.1016/s0378-5955(02)00336-2. [DOI] [PubMed] [Google Scholar]
- Wysocki LE, Ladich F. The representation of conspecific sounds in the auditory brainstem of teleost fishes. J. Exp. Biol. 2003;206:2229–2240. doi: 10.1242/jeb.00417. [DOI] [PubMed] [Google Scholar]
- Yost WA. Fundamentals of Hearing—An Introduction. 4. San Diego: Academic Press; 2000. [Google Scholar]