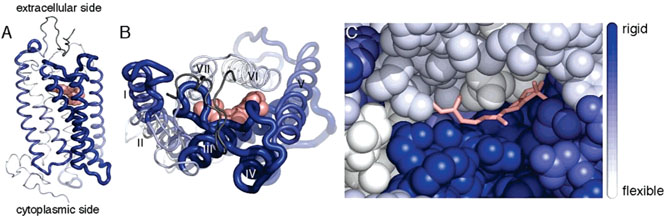
Figure 6.
Rigidity of structural segments of bovine rhodopsin. The rigidity, κ, of structural segments is mapped onto the crystal structure of bovine rhodopsin (PDB ID 1U1967). Segments are colored from white to blue according to their rigidity, where white represents the most flexible region (κ = 0.9 N/m) and blue represents the most rigid region (κ = 4.2 N/m). The rigidity of segments is also indicated by the thickness of the chain, where increasing thickness corresponds to increasing rigidity. The first 19 amino acid residues are dark gray because no rigidity value is available for that region. (A) Side view of rhodopsin with the extracellular side on the top and the cytoplasmic side on the bottom. 11-cis-Retinal is represented as pink spheres. (B) Top view of rhodopsin from the extracellular side looking down into the retinal-binding pocket. (C) Enlarged view of B with rhodopsin represented as spheres and 11-cis-retinal displayed in a stick representation. The amino terminus and extracellular loops have been removed in this image so that the chromophore is visible. Extracellular loop II forms a lid over the retinal-binding pocket and would cover the chromophore in this view. Images were created in PyMOL v. 0.99.68