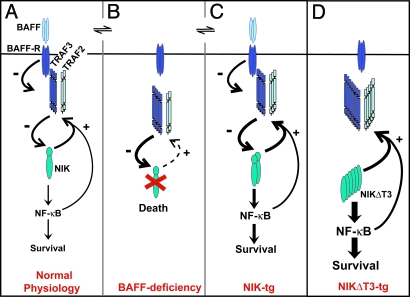
Fig. 5.
Scheme of BAFF:BAFF-R-induced alternative NF-κB activation via TRAF2, TRAF3, and NIK. During normal B cell physiology (A), BAFF:BAFF-R interactions induce the degradation of TRAF3, which binds to NIK at the T3BD and together with TRAF2 negatively regulates NIK protein levels. NIK activity stimulates B cell survival via the activation of predominantly alternative NF-κB and increases the TRAF2 and, more prominently, TRAF3 protein levels in a negative feedback interaction. (B) In the absence of BAFF:BAFF-R interactions, TRAF3 accumulates and together with TRAF2 induces the complete degradation of NIK, leading to shutdown of alternative NF-κB activity and cellular apoptosis. (C) In the presence of BAFF, increased expression of NIK leads to a steady state of enhanced NF-κB signaling and B cell survival and enhanced levels of TRAF2 and TRAF3, which keep NIK protein level below detection. Absence of BAFF (B) still results in TRAF2/3-mediated degradation of NIK and apoptosis. The activity of increased de novo generated NIK mostly likely slightly increases BAFF-independent survival. (D) NIKΔT3 induces BAFF-independent maximal p100 processing and B cell survival and very high TRAF2 and TRAF3 protein levels, which, however, cannot impact on NIKΔT3 protein levels.