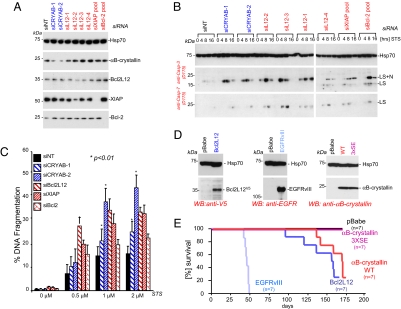
Fig. 2.
αB-crystallin is a potent antiapoptotic protein in human glioma cells. (A) LN235 cells were transfected with RNAi oligonucleotides (each 100 nM) targeting αB-crystallin (siCRYAB-1 and siCRYAB-2), Bcl2L12 (siL12–1 to siL12–4), XIAP, and Bcl-2 (siRNA pools) and subjected to Western blot analysis to assess levels of protein knockdown. The migration positions of αB-crystallin, Bcl2L12, XIAP, and Bcl-2 are indicated. Hsp70 is shown as a loading control. (B) LN235 cells transfected with the indicated RNAi oligonucleotides were treated with STS (2 μM) for 0, 4, 8, and 16 h and subjected to Western blot analysis to assess effector caspase activation status. The migration positions of active caspase-3 and active caspase-7 species are indicated. Hsp70 protein levels are shown as a loading control. (C) LN235 transfectants were treated with the indicated doses of STS for 24 h, and DNA fragmentation was assessed by quantification of subG1 DNA peaks. Mean values of three to four independent experiments are shown with error bars representing SDs, and P values were calculated by using Student's t test. (D) Western blot analysis of LN443 cells retrovirally transfected with Bcl2L12V5, EGFRvIII, αB-crystallin WT, and 3XSE point mutants. The migration positions of Bcl2L12, EGFRvIII, αB-crystallin and Hsp70 (loading control) are indicated. (E) Kaplan-Meier curves of SCID animals intracranially injected with LN443 cells retrovirally transduced with pBabe control, αB-crystallin WT, and 3XSE, Bcl2L12V5 and EGFRvIII. ID50 and P values (vs. pBabe): WT (167 days, P = 0.046), 3XSE (not determined), Bcl2L12 (157 days, P = 0.0042), EGFRvIII (46 days, P < 0.001). P values were calculated by using logrank test.