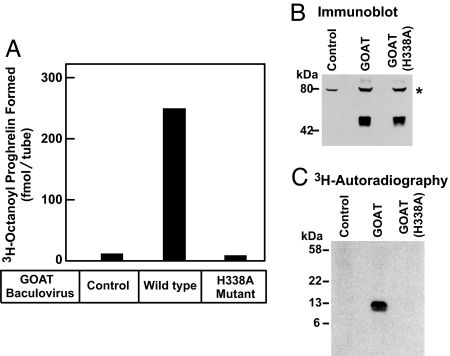
Fig. 1.
Establishment of in vitro octanoylation assay. (A) GOAT activity in membranes from Sf9 insect cells. On day 0, Sf9 cells were set up for experiments and infected on day 1 with baculovirus encoding wild-type GOAT or the H338A-mutant GOAT, both containing a N-terminal His10 tag. After infection for 48 h, the 100,000 × g membrane fraction from the uninfected cells (control) and the infected cells were prepared as described in Experimental Procedures. Each 50-μl reaction mixture contained 50 μg of membrane protein from the indicated cells, 5 μg of proghrelin-His8, 50 μg of myristyl ether CoA, and 1 μM [3H]octanoyl CoA (11 dpm/fmol). After incubation for 5 min at 37°C, the amount of [3H]octanoyl transferred to proghrelin-His8 was quantified by nickel chromatography and scintillation counting as described in Experimental Procedures. Each value represents the average of triplicate assays. (B) Immunoblot analysis of GOAT expressed in Sf9 cells. Aliquots of the membranes used in A (75 μg of protein) were subjected to immunoblot analysis with 1 μg/ml monoclonal anti-His antibody. The asterisk denotes an irrelevant cross-reacting band present in the membranes of Sf9 cells. Film was exposed for 10 s. (C) Autoradiography of [3H]octanoyl proghrelin-His8 formed in the in vitro assay. The reaction products from replicate assays in A were precipitated with 80% acetone, loaded onto 16% Tricine SDS/PAGE, transferred to a PVDF membrane, and subjected to autoradiography for 5 days as described in SI Experimental Procedures.