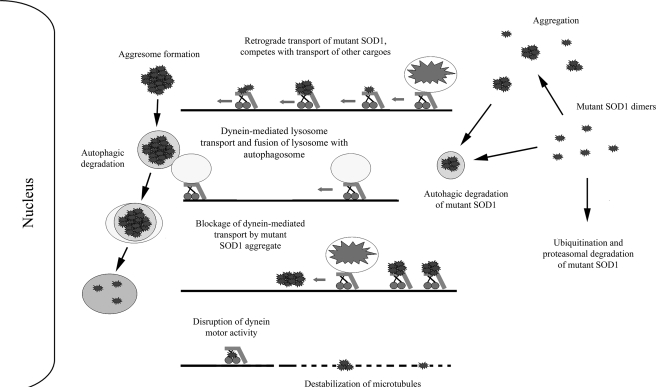
FIGURE 8.
Potential role(s) of the dynein-dynactin motor complex in mutant SOD1-mediated ALS. Dynein-dynactin can sequester and transport mutant SOD1 to form large aggresome-like inclusions that require autophagic degradation. Dynein is also involved in the autophagosome-lysosome fusion that is critical to the degradation of misfolded protein inclusions in autophagosomes. Interplay of the two dynein-dependent events, the formation and degradation of inclusions, determines the eventual inclusion burden in neurons. Increased levels of mutant SOD1 associated with dynein as the disease progresses could contribute to decreased axonal transport in ALS by multiple mechanisms. These potential mechanisms illustrated here include competitive occupancy of the dynein-mediated transport capacity, disruption of dynein motor activity, destabilization of microtubules, physical blockage of transport by mutant SOD1 aggregates in the axon. Decreased retrograde transport of cargos such as mitochondria and neurotrophic factors as well as increased aggregation burden may determine the toxicity of mutant SOD1 and ALS disease manifestation.