Figure 1.
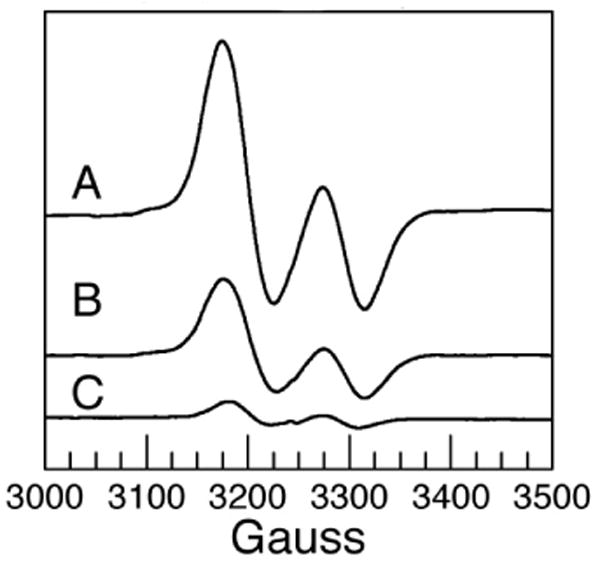
EPR spectra of steady-state 2-aminopropanol radicals at the active site of ethanolamine ammonia-lyase. Ethanolamine ammonia lyase and adenosylcobalamin were mixed with 2-aminopropanol, and frozen by dipping in liquid nitrogen. A (S)-2-aminopropanol. B (R)-2-aminopropanol. C [1-2H2]-(R)-2-aminopropanol. Spectra were recorded at 77 K. The ordinate is scaled such that amplitudes are proportional to concentration. Samples contained 0.24 mM enzyme, 0.47 mM adenosylcobalamin, 25 mM 2-aminopropanol, and 0.01 M Hepes/NaOH pH 7.5.
