Fig. 1.
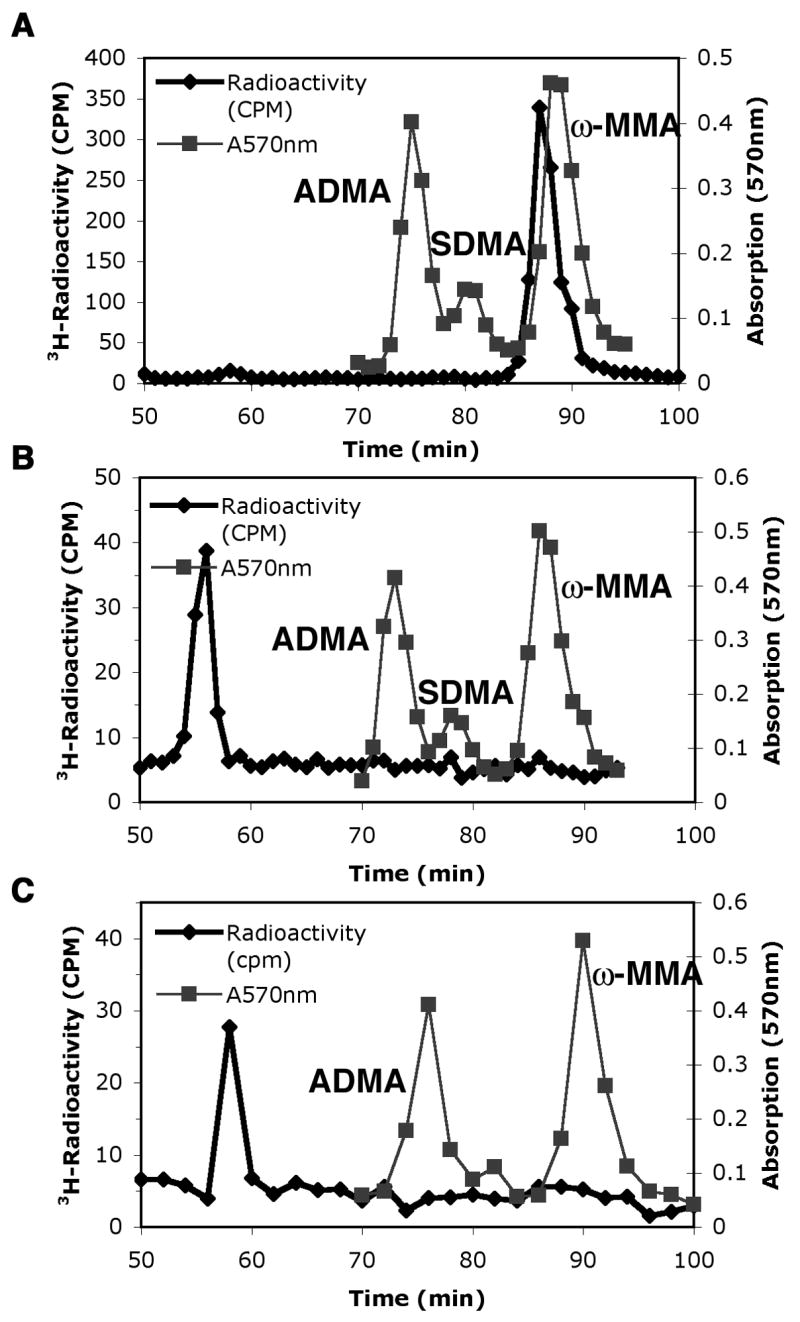
Predominant type III protein arginine methyltransferase activity of Hsl7 with calf thymus histone H2A. Amino acid analysis of 3H-methylated arginine residues when histone H2A was incubated for 1 h at 37 °C with wild type GST-Hsl7 (panel A) or mutant GST-Hsl7G387A (panel B) in the presence of 3H-AdoMet as described in the “Materials and methods” section. A control is also shown when the wild type enzyme is incubated in the absence of histone H2A (panel C). 3H-Radioactivity from mixing 200 μl of each fraction with 400 μl water and 5 mL scintillation fluor (Safety Solve, Research Products International, Mt. Prospect, IL) is seen in the black line with diamonds. Unlabeled standards of ω-MMA, ADMA, and SDMA were detected with a ninhydrin assay using 100 μl of each fraction [16] and are shown as absorption at 570 nm by gray lines with squares. As expected, the elution position of the tritiated methyl arginine derivatives is slightly ahead of the non-isotopically labeled standards [17].
