Abstract
In humans, partial-thickness cartilage lesions frequently result in premature osteoarthritis. While rabbits often are used as a model for partial-thickness cartilage lesions, the natural course of cartilage surrounding such a lesion is largely unknown. We developed a rabbit model of a chronic partial-thickness cartilage defect and asked whether these defects led to (1) deterioration of surrounding cartilage macroscopically and microscopically (increased Mankin score) and (2) disturbances in proteoglycan metabolism. In 55 rabbits, we created a 4-mm-diameter partial-thickness cartilage defect on one medial femoral condyle. The surrounding cartilage was characterized during the course of 26 weeks. Contralateral knees were sham-operated. In experimental knees, we found cartilage softening and fibrillation at 13 and 26 weeks. High Mankin scores observed at 1 week were partially restored at 13 weeks but worsened later and were most pronounced at 26 weeks. Mankin scores in the experimental groups were worse at 1 and 26 weeks when compared with the sham groups. Mankin scores at 26 weeks improved compared with 1 week in the sham groups. Disturbances in proteoglycan metabolism were less evident. In this rabbit model, a partial-thickness cartilage lesion resulted in early markers of degenerative changes resembling the human situation.
Introduction
Clinically, preventing joint degeneration is an important rationale for repairing cartilage defects. Early diagnosis and treatment of patients are recommended to prevent progression to advanced osteoarthritis (OA). However, most chondral defects are not symptomatic [5, 9] and therefore exist for some time before they are treated. Furthermore, the period of preoperative symptoms before repair of the chondral defect often is extended. In our clinic, the mean duration of symptoms before a defect was treated was 29 months (range, 4–48 months) and 23 months (range, 3–48 months) for perichondrium transplantation and open débridement and drilling, respectively [2]. In one study, the mean duration of symptoms for treating osteochondritis dissecans by autologous chondrocyte transplantation was 7.8 years (range, 0.1–36 years) [25]. The extended duration between the occurrence of a cartilage defect and its treatment in humans will likely negatively influence the outcome of cartilage repair owing to changes in joint homeostasis [27]. In contrast to the human setting, in some animal studies, the cartilage lesions were treated immediately after creation of the defect [7, 28]. The discrepancies between successfully tested cartilage repair techniques in animals and the less favorable outcomes in patients could be explained by the chronic disturbances in human joint homeostasis relating to the delay in treatment [2, 3, 13]. Therefore, an animal model that better reflects the clinical situation, including an extended period of preoperative cartilage damage [27], would be better suited for evaluating experimental cartilage repair techniques.
Full-thickness cartilage defects smaller than 3 mm in diameter in a rabbit model reportedly regenerate spontaneously [23]. However, few studies have focused on alterations in cartilage surrounding a partial-thickness articular cartilage defect. Lu et al. [18] reported ongoing degeneration of cartilage surrounding the defect in a sheep model during the course of 52 weeks. Because rabbits often are used as a model to test novel cartilage repair techniques, Hunziker and Quinn [14] reported a considerable number of chondrocytes were lost from cartilage adjacent to surgically created partial-thickness articular cartilage defects, whereas the synthetic activity of the remaining chondrocytes remained unchanged. However, those authors created cartilage lesions with a width of 1 mm, which we believe is too small for testing current cartilage repair techniques.
We describe a rabbit model in which chronic partial-thickness articular cartilage defects were created with a diameter of 4 mm. We asked whether these lesions led to (1) deterioration of surrounding cartilage macroscopically (loss of glossy appearance) and microscopically (increased Mankin scores) and (2) disturbances in proteoglycan (PG) metabolism (increase in PG synthesis rate and inability of the cartilage matrix to retain newly synthesized PGs) reflecting degenerating articular cartilage.
Materials and Methods
We followed macroscopic, histologic, and biochemical changes during the course of 26 weeks to reflect degenerative changes in articular cartilage surrounding 4-mm defects created on the medial femoral condyles of 55 3-month-old New Zealand White rabbits (females; average weight, 2.5 kg; range, 1.5–3.0 kg). Contralateral knees were sham-operated. From previous experiments and the literature [21, 27], the sample size was determined based on a difference of 1.5 points in the Mankin score (as described by Mankin et al. [19]) using the power analysis of Sachs [26], with a power of 80%, two-tailed, and a confidence interval of 95%. This resulted in a minimum of five knees per group for histologic evaluation. For analysis of early changes in PG synthesis rate and ability of the cartilage matrix to retain newly synthesized PGs, 12 knees per group for each followup were chosen [21]. Two rabbits were excluded from analysis; one rabbit died of pneumonia and the second had a knee infection. One sample was lost (Table 1). The experiments were conducted following the national and European guidelines for animal experiments. The Maastricht University Committee for Animal Experiments approved all experimental protocols.
Table 1.
Rabbit demographics
| Followup (weeks) | Number of rabbits | Weight (kg)* | Number of knees | |||
|---|---|---|---|---|---|---|
| Histology | Biochemistry | |||||
| Sham | Defect | Sham | Defect | |||
| 1 | 18 | 2.6 (0.12) | 6 | 5 | 12 | 12 |
| 13 | 17 | 3.7 (0.46) | 5 | 5 | 12 | 12 |
| 26 | 18 | 4.4 (0.44) | 6 | 6 | 12 | 12 |
* Values are expressed as means, with standard deviations in parentheses.
Preoperatively, each rabbit was fasted for 12 hours. General anesthesia was induced by intramuscular injection of 35 mg ketamine hydrochloride per kg body weight and 5 mg xylazine per kg and maintained throughout the surgical procedure by administration of 2% halothane and a mixture of oxygen and nitrous oxide delivered by an automatic ventilator using a specially designed mask. Preoperatively, all rabbits received an intramuscular injection of 10 mg ceftiofur per kg. Arthrotomy of the tibia-femoral articulation was performed through a medial longitudinal parapatellar incision. The patella was dislocated laterally to expose the surface of the medial femoral condyle. A 4-mm-diameter skin biopsy punch (KAI Europe GmbH, Solingen, Germany) was used to circumscribe the defect centered on the weightbearing part of the medial femoral condyle. Noncalcified cartilage was removed from the outlined defect using a scalpel (defect group) to create a partial-thickness defect. Special care was taken to prevent penetration of the subchondral bone. The contralateral knees (sham group) received an arthrotomy followed by lateral dislocation of the patella, as performed in the experimental knees but without creation of a defect. At the end of the procedure in the defect and the sham groups, the patellae were relocated and the wound was closed in layers. Postoperative pain relief was provided by administering 50 μg buphenomorphine per kg at 2 hours and 1 day. The rabbits were housed in a cage for 2 days, after which they were allowed to have unlimited activity in groups in a stable. They were fed a standard rabbit diet and had water ad libitum. They were euthanized 1, 13, and 26 weeks after surgery with an overdose of pentobarbital. For macroscopic purposes, the femoral condyles were dissected and photographed. The lesions were evaluated for whether they were healed, and cartilage of the medial femoral condyles was examined for cartilage softness and fibrillation (indicated by a loss of glossy appearance). Then condyles were prepared either for histologic grading or for determination of PG synthesis and PG retention capacity of the cartilage surrounding the defects using [35S]sulfate incorporation ex vivo.
For histologic analysis, condyles were fixed in a 10% formalin solution for 5 days at 4° C. After decalcification in a 10% EDTA solution, samples were dehydrated in a series of increasing concentrations of ethanol and embedded in 2-hydroxyethyl methacrylate (Technovit 7100; Heraeus Kulzer GmbH, Wehrheim, Germany). Sections of 5 μm were cut along the midsagittal plane using a multirange microtome (LKB, Stockholm, Sweden) and stained with thionine. All sections were viewed at the same time by two individuals (EJJ, RK) who were blinded to group assignment. The articular cartilage on the entire width of the medial femoral condyle was evaluated using the histologic and histochemical grading system of Mankin et al. [19]. Lower scores indicate better histologic appearance.
For biochemical analysis, the cartilage was harvested from the medial femoral condyles under aseptic conditions and transferred to preweighed tubes containing 1 mL medium (Dulbecco’s Modified Eagle’s Medium/Ham’s F12 nutrient mix with GlutaMAX™ I; Invitrogen, Breda, The Netherlands) supplemented with ascorbic acid 2-phosphate (0.2 mmol/L; Sigma-Aldrich Chemie BV, Zwijndrecht, The Netherlands), penicillin (100 U/mL), streptomycin (100 μg/mL), and amphotericin (0.25 μg/mL) (Invitrogen). In the defect group, the cartilage 2 to 3 mm proximal and distal of the created defect was dissected. The medium was discarded and 500 μL medium supplemented with 3.7 × 105 Bq Na352SO4 (Amersham Biosciences Benelux, Roosendaal, The Netherlands) ([35S]sulfate medium) per mL was added. The samples were incubated in a humidified CO2 incubator overnight. The [35S]sulfate medium was removed and cartilage samples were washed for 10 minutes three times with 1 mL sterile phosphate-buffered saline. One half of the samples were used for analysis of the PG synthesis [16] and the other half for analysis of the PG retention capacity. To determine PG retention, cartilage samples were cultured under normal conditions in the presence of 10% fetal bovine serum for an additional 48 hours [24]. The cartilage samples were completely digested in a solution containing 0.15 μg proteinase K (Merck-Europe BV, Amsterdam, The Netherlands) per μL, 0.1 μg PG (A1 fraction isolated from human articular cartilage) per μL, 50 mmol Tris–HCl (pH 7.9) per L, and 1 mmol CaCl2 (Merck-Europe BV) per L in a shaking water bath at 56° C for 3 days. After centrifugation at 3000 g for 5 minutes, the DNA content of the supernatants was assessed using a commercially available assay kit (CyQUANT® DNA assay kit; Invitrogen) according to the manufacturer’s instructions. In brief, 200 μL CyQUANT® GR dye/cell lysis buffer was added to each sample. An aliquot of each sample was incubated for 5 minutes at room temperature protected from light exposure. The sample fluorescence was measured at 480-nm excitation and 520-nm emission wavelengths. Fluorescence measurements were compared with the values obtained from a standard DNA curve, and the resulting DNA content was normalized to the cartilage wet weight.
The remaining supernatant was supplemented with cetylpyridinium chloride (Merck-Europe BV) and NaCl to a final concentration of 0.5% (w/v) and 0.2 mol/L, respectively. Samples were incubated at 37° C for 1 hour to precipitate the glycosaminoglycans, which then were centrifuged at 15,000 g for 5 minutes. The supernatants were discarded and the pellets were washed once with 100 μL of a solution of 0.1% cetylpyridinium chloride in 0.2 mol NaCl per L and then dried. Pellets were dissolved in 100 μL formic acid (Merck-Europe BV) at room temperature for 24 hours. A 10-μL aliquot of each sample was mixed with 2.5 mL Formula 989 scintillation fluid (DuPont, Dordrecht, The Netherlands) and counted in a liquid scintillation counter. The total [35S]sulfate incorporation of each cartilage sample was calculated using the specific activity of the medium and was normalized to the cartilage wet weight.
Data were not normally distributed and therefore were analyzed using nonparametric tests. First, data were analyzed using the Kruskal-Wallis (nonparametric one-way analysis of variance) and Friedman overall tests. Then, a two-tailed Mann-Whitney U test was performed to compare differences in Mankin score, including all its individual parameters (structure, cells, matrix, tidemark) and PG metabolism (PG synthesis and PG retention capacity) between treated (having a previously created cartilage lesion) and sham-treated knees. A p value less than 0.05 was considered significant. All data were analyzed with SPSS Version 12.0.1 (SPSS Inc, Chicago, IL).
Results
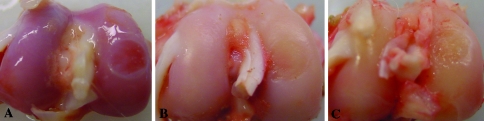
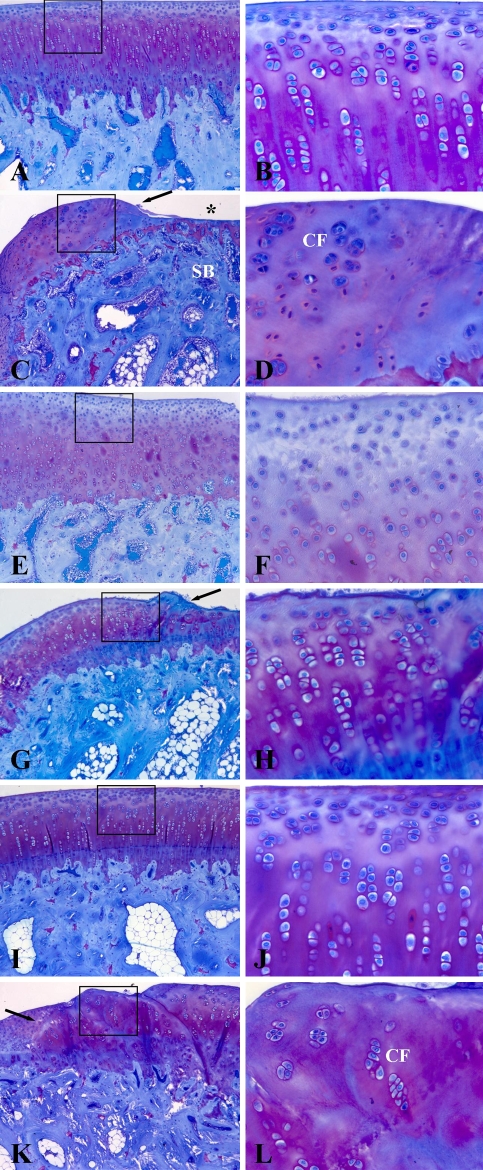
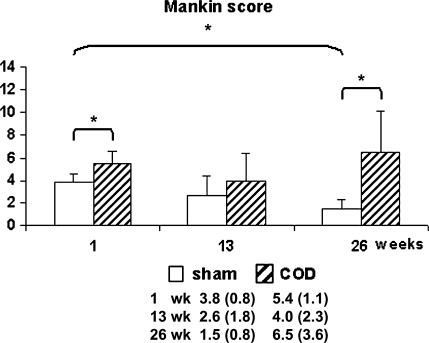
The creation of partial-thickness articular cartilage lesions resulted in changes suggesting early degeneration: cartilage softening and fibrillation, indicated by loss of glossy appearance of the articular surface at 13 and 26 weeks. These changes were confirmed by histologic analysis (increased Mankin scores) and biochemical alterations (changes in PG metabolism). At 1 week (Fig. 1A), the cartilage surrounding the defect had a glossy, white, smooth appearance, which disappeared at 13 (Fig. 1B) and 26 weeks (Fig. 1C). In addition, at 13 and 26 weeks, the articular surface around the created lesion showed signs of fibrillation. In the sham groups, no noticeable macroscopic abnormalities were observed during the course of 26 weeks (not shown), but histologic analysis revealed minor degenerative changes (Fig. 2). We found higher Mankin scores in the defect groups at 1 week (p = 0.030) and 26 weeks (p = 0.024) compared with the sham groups (Fig. 3). At 1 week, the sham group (Fig. 2A–B) scored better (p = 0.036) than the defect group (Fig. 2C–D) on the structure parameter (1.0 versus 2.0 for the sham and defect groups, respectively) (Table 2). Surface irregularities were more pronounced in the defect group compared with the sham group. Cartilage cellularity was similar between sham and defect knees. In both groups, the matrix staining was reduced compared with the lateral femoral condyle and the tidemark integrity was disturbed. At 13 weeks, we observed no differences between the groups. The articular surface remained irregular, whereas cartilage cellularity was normal in both groups. Reduced matrix staining and tidemark abnormalities also were observed at 13 weeks (Fig. 2E–H). At 26 weeks, the sham group scored better than the defect group in the structure (p = 0.022), cells (p = 0.022), and tidemark parameters (p = 0.027) (Table 2). In the defect group (Fig. 2K–L), the surface showed more clefts compared with the sham group (Fig. 2I–J). We observed cell clusters embedded in slightly stained matrix, whereas the tidemark integrity remained disturbed. In the sham series, the histologic appearance of the cartilage at 26 weeks improved (p = 0.005) compared with 1 week (Fig. 3). The matrix (p = 0.004) and tidemark parameters (p = 0.027) also were improved (Table 2). The mean cartilage wet weights and DNA contents of defect- and sham-treated knees were similar (Table 3).
Fig. 1A–C.
Representative photographs are shown of articular surfaces at (A) 1 week, (B) 13 weeks, and (C) 26 weeks after creating partial-thickness articular cartilage defects on rabbit medial femoral condyles. Cartilage surrounding the defect had a glossy, white, smooth appearance at 1 week, which disappeared during the course of 26 weeks.
Fig. 2A–L.
Photomicrographs of sections are shown at (A–D) 1 week, (E–H) 13 weeks, and (I–L) 26 weeks. (A) A sham-treated rabbit medial femoral condyle at 1 week followup (Stain, thionine; original magnification, ×100); (B) an enlargement of the box in (A) (Stain, thionine; original magnification, ×400); and (C) a condyle with partial-thickness articular cartilage defect at 1 week followup are shown (Stain, thionine; original magnification, ×100). The cartilage defect (*) did not penetrate the subchondral bone (SB); (D) An enlargement of the box in (C) is shown (Stain, thionine; original magnification, ×400). A cluster formation (CF) can be seen. (E) A sham-treated rabbit medial femoral condyle at 13 weeks followup (Stain, thionine; original magnification, ×100); (F) an enlargement of the box in (E) (Stain, thionine; original magnification, ×400); (G) a condyle with a partial-thickness articular cartilage defect at 13 weeks followup (Stain, thionine; original magnification, ×100); and (H) an enlargement of the box in (G) are shown (Stain, thionine; original magnification, ×400). (I) A sham-treated rabbit medial femoral condyle at 26 weeks followup (Stain, thionine; original magnification, ×100); (J) an enlargement of the box in (I) (Stain, thionine; original magnification, ×400); and (K) a condyle with partial-thickness articular cartilage defect at 26 weeks followup are shown (Stain, thionine; original magnification, ×100). The partial-thickness articular cartilage defect was not healed at 26 weeks. Cartilage surrounding the defect showed surface irregularities; (L) an enlargement of the box in (K) is shown (Stain, thionine; original magnification, ×400). A cluster formation (CF) can be seen. The arrows in (C), (G), and (K) indicate the edge of the defect.
Fig. 3.
A histogram shows the degree of degenerative changes (using the Mankin score) in rabbit medial femoral condyles 1, 13, or 26 weeks postoperatively. Worse histologic appearances were observed in the defect (COD) groups at 1 and 26 weeks when compared with the sham groups. The key shows means and standard deviations. * = significant at p < 0.05.
Table 2.
Mankin scores [19] at the different followup times
| Mankin parameter | 1 Week | 13 Weeks | 26 Weeks | |||
|---|---|---|---|---|---|---|
| Sham | Defect | Sham | Defect | Sham | Defect | |
| Structure | 1.0 (0.0) | 2.0 (1.2) | 1.2 (0.4) | 1.8 (1.8) | 1.0 (0.0) | 2.8 (1.8) |
| Cells | 0.0 (0.0) | 0.8 (1.3) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 1.7 (1.4) |
| Matrix | 2.0 (0.6) | 1.6 (0.9) | 0.8 (1.1) | 1.2 (0.8) | 0.3 (0.5) | 1.2 (1.0) |
| Tidemark | 0.8 (0.4) | 1.0 (0.0) | 0.6 (0.5) | 1.0 (0.0) | 0.2 (0.4) | 0.8 (0.4) |
Values are expressed as means, with standard deviations in parentheses.
Table 3.
Data on metabolic properties of articular cartilage at the different followup times
| Followup (weeks) | Wet weight (μg) | DNA content (ng/μg) | PG synthesis (dpm/μg) | PG retention capacity (dpm/μg) | ||||
|---|---|---|---|---|---|---|---|---|
| Sham | Defect | Sham | Defect | Sham | Defect | Sham | Defect | |
| 1 | 6.7 (3.6) | 7.3 (5.0) | 2.1 (1.9) | 2.1 (1.4) | 2646 (1961) | 2615 (1959) | 814 (504) | 270 (110) |
| 13 | 5.6 (3.2) | 7.0 (2.9) | 3.2 (2.7) | 1.6 (0.5) | 1915 (1560) | 1237 (383) | 423 (306) | 295 (122)* |
| 26 | 8.4 (2.6) | 8.8 (4.8) | 0.8 (0.3) | 1.4 (1.1) | 1039 (271) | 1103 (590) | 339 (223) | 175 (90)* |
Values are expressed as means, with standard deviations in parentheses; *significantly different at p < 0.05; PG = proteoglycan.
Between 13 and 26 weeks, we found a decrease (p = 0.045) in the PG retention capacity in defect-treated knees. However, we observed no differences in cartilage metabolism (PG synthesis and PG retention capacity) between cartilage from defect- and sham-treated knees at 1, 13, and 26 weeks (Table 3).
Discussion
In humans, untreated articular cartilage lesions often progress toward premature OA. This rationale for recent trends for early repair is to prevent the OA. Numerous novel repair procedures have been and are being developed for this purpose. Such procedures often are tested in animal models usually in symptom-free joints, whereas isolated lesions in patients can be present for a considerable time before treatment occurs. The natural course of cartilage surrounding an isolated cartilage lesion in the often-used acute rabbit model is largely unknown and may not resemble the clinical setting with chronic alterations. Therefore, we evaluated the effect of the lesion with time on the surrounding cartilage with regard to macroscopic (softening and fibrillation), microscopic (increased Mankin score), and biochemical (increased PG synthesis and inability to retain newly synthesized PGs) parameters.
This study has two major limitations. First, while economically and practically attractive, the rabbit model is not an entirely suitable animal model to study articular cartilage repair procedures in preclinical studies [13]. Hunziker [13] noted “...the matrix domain sustained and remodeled by an individual cellular unit is, in the human, approximately 8 to 10 times larger than that in the rabbit.” This likely would lead to substantial enhancement in the rabbit to maintain surrounding cartilage compared with the human. Nevertheless, the rabbit is probably the most often used model for economic reasons and the literature contains interpretations based on rabbit data. Although we believe our delayed rabbit model better represents the clinical situation, cartilage repair procedures using this model should still be interpreted with caution before proceeding to clinical studies. The second limitation is the power of the study, which was sufficient for histologic grading using the Mankin score but not sufficient for the biochemical parameters studied. Thus, we can describe only trends for the PG synthesis and the capacity of the cartilage matrix to retain newly synthesized PGs.
In this model, partial-thickness articular cartilage lesions with a 4-mm diameter did not heal during the course of 26 weeks and we found no signs of regeneration; these lesions therefore represent critical-size defects. We observed degenerative features macroscopically and microscopically at 13 and 26 weeks around the cartilage defects. Histologically, the degeneration observed 1 week postoperatively was partially reversed at 13 weeks but then tended to increase again from 13 to 26 weeks. We noted no progressive cartilage degeneration (as reflected by the Mankin scores) from 13 to 26 weeks. This probably is attributable to the slow progression of the degenerative process, as has been observed in other quadrupeds. In the dog, severe degeneration is first evident 5 years after initiation of the process [4]. The time at which severe degeneration occurs in rabbits is unknown.
The sham-treated knee showed articular cartilage changes during the first weeks after the arthrotomy, which can be explained by the effects of the operation (e.g., effect of exposure of room air, stress of the sutures in the relatively small joint [1, 8, 22, 30]). During the course of 26 weeks, the cartilage appeared to fully recover as observed histologically. This suggests regeneration of the mild changes in sham-operated knees, which may be related to peculiarities of the rabbit model.
Cell density was diminished at the wound edge of the cartilage defects as observed histologically. However, we observed normal cellularity using biochemical measurements. These findings were consistent with results described by Hunziker and Quinn [14], who reported with quantitative autoradiographic analysis chondrocytes within 100 μm of a partial-thickness defect had synthetic activity similar to that of cells far from the lesion. Furthermore, the DNA assay, although one of the best available yet, may not be sensitive enough to detect the cell death occurring in the edges of the cartilage defects. Therefore, normal cellularity, or even increased cell numbers, in remote areas might compensate for hypocellularity in the wound edges.
Biochemically, cartilage from experimental knees did not differ from cartilage in sham-treated knees, which could indicate cartilage surrounding a partial-thickness articular defect was biochemically normal. However, this is in contrast to our histologic findings suggesting degenerative changes during the course of 26 weeks after creating the defect. We noted a nonsignificant trend toward persistent loss in PG retention after creation of the defect and this might have contributed to the observed histologic degeneration. A possible explanation for this lack of difference is that in sham-treated knees persistent biochemical alterations take place in the first weeks because of the arthrotomy, as was observed histologically, and after 13 weeks because of alterations in joint homeostasis, occurring before histologic changes in cartilage degeneration.
Our model involves creation of one circumscribed partial-thickness articular cartilage lesion without concomitant injuries of the meniscus or anterior cruciate ligament. It has advantages compared with other animal models: (1) when similar diameters are used, the effect of cartilage repair techniques can be monitored without the confounding effects of other potential causes of cartilage degeneration; (2) the operation is relatively simple and creates circumscribed cartilage lesions; (3) repair of these chronic partial-thickness articular cartilage lesions occurs with surrounding degeneration, which resembles the clinical situation [9, 12, 17, 23, 29, 32]; and (4) cartilage lesions are created on the medial femoral condyle, which is the most commonly affected zone of articular cartilage damage observed with arthroscopies in humans [6, 10, 11]. Existing animal models intended to replicate human OA fail to resemble the clinical situation of a focal cartilage lesion, whereas the permanent trigger for degeneration will interfere with attempts of cartilage repair or regeneration [15, 31]. Damaging articular cartilage, as described in the groove model [20, 21], did not reflect a one-time trauma in the clinical setting. Furthermore, it would be challenging to reproduce exactly the same grooves in each animal as far as depth and length. Penetration of the subchondral bone, as described in the articular step-off model [17], allows migration of mesenchymal stem cells influencing the repair process. In addition, although these are models for an advanced stage of OA, they are not expected to reflect the altered matrix metabolism and articular cartilage degeneration surrounding a focal partial-thickness articular cartilage lesion with time.
We report the evolution of cartilage changes surrounding a partial-thickness articular cartilage defect in a rabbit model during the course of 26 weeks. We believe a delayed treatment model is important when exploring cartilage repair strategies to prevent degenerative changes. Our data suggest a defect at least 13 weeks old most likely resembles the clinical focal cartilage lesion that has failed to heal after an initial remodeling process.
Acknowledgments
We thank Joyce Suyk, Monique de Jong, Frans Slangen, and May Bost from the Central Experimental Animal Facility of Maastricht University for assistance in surgery and animal care. The laboratory assistance of Don Surtel, Martine Hulsbosch, and Mireille Schrooten-van Helden is gratefully acknowledged. We also thank Els Terwindt-Rouwenhorst and Paul van Dijk from the Department of Anatomy and Embryology for technical assistance and Nick Guldemond for assistance with the statistical analysis.
Footnotes
Each author certifies that he has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Bauer MS, Woodard JC, Weigel JP. Effects of exposure to ambient air on articular cartilage of rabbits. Am J Vet Res. 1986;47:1268–1270. [PubMed]
- 2.Bouwmeester SJ, Kuijer R, Homminga GN, Bulstra SK, Geesink RG. A retrospective analysis of two independent prospective cartilage repair studies: autogenous perichondrial grafting versus subchondral drilling 10 years post-surgery. J Orthop Res. 2002;20:267–273. [DOI] [PubMed]
- 3.Bouwmeester SJ, Kuijer R, Terwindt-Rouwenhorst EA, van der Linden AJ, Bulstra SK. Histological and biochemical evaluation of perichondrial transplants in human articular cartilage defects. J Orthop Res. 1999;17:843–849. [DOI] [PubMed]
- 4.Brandt KD, Myers SL, Burr D, Albrecht M. Osteoarthritic changes in canine articular cartilage, subchondral bone, and synovium 54 months after transaction of the anterior cruciate ligament. Arthritis Rheum. 1991;34:1560–1570. [DOI] [PubMed]
- 5.Bredella MA, Tirman PF, Peterfy CG, Zarlingo M, Feller JF, Bost FW, Belzer JP, Wischer TK, Genant HK. Accuracy of T2-weighted fast spin-echo MR imaging with fat saturation in detecting cartilage defects in the knee: comparison with arthroscopy in 130 patients. AJR Am J Roentgenol. 1999;172:1073–1080. [DOI] [PubMed]
- 6.Curl WW, Krome J, Gordon ES, Rushing J, Paterson Smith B, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13:456–460. [DOI] [PubMed]
- 7.Gao J, Knaack D, Goldberg VM, Caplan AI. Osteochondral defect repair by demineralized cortical bone matrix. Clin Orthop Relat Res. 2004;427(suppl):S62–S66. [DOI] [PubMed]
- 8.Han CD, Kang HJ. Changes in the hyaline articular cartilage after air exposure. Yonsei Med J. 1990;31:53–59. [DOI] [PubMed]
- 9.Hardaker WT Jr, Garrett WE Jr, Bassett FH 3rd. Evaluation of acute traumatic hemarthrosis of the knee joint. South Med J. 1990;83:640–644. [DOI] [PubMed]
- 10.Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18:730–734. [DOI] [PubMed]
- 11.Hunt N, Sanchez-Ballester J, Pandit R, Thomas R, Strachan R. Chondral lesions of the knee: a new localization method and correlation with associated pathology. Arthroscopy. 2001;17:481–490. [DOI] [PubMed]
- 12.Hunziker EB. Articular cartilage repair: basic science and clinical progress: a review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–463. [DOI] [PubMed]
- 13.Hunziker EB. Biologic repair of articular cartilage: defect models in experimental animals and matrix requirements. Clin Orthop Relat Res. 1999;367(suppl):S135–S146. [DOI] [PubMed]
- 14.Hunziker EB, Quinn TM. Surgical removal of articular cartilage leads to loss of chondrocytes from cartilage bordering the wound edge. J Bone Joint Surg Am. 2003;85(suppl 2): 85–92. [DOI] [PubMed]
- 15.Johnson JM, Johnson AL. Cranial cruciate ligament rupture: pathogenesis, diagnosis, and postoperative rehabilitation. Vet Clin North Am Small Anim Pract. 1993;23:717–733. [DOI] [PubMed]
- 16.Lafeber FP, Vander Kraan PM, Van Roy JL, Huber-Bruning O, Bijlsma JW. Articular cartilage explant culture: an appropriate in vitro system to compare osteoarthritic and normal human cartilage. Connect Tissue Res. 1993;29:287–299. [DOI] [PubMed]
- 17.Lefkoe TP, Trafton PG, Ehrlich MG, Walsh WR, Dennehy DT, Barrach HJ, Akelman E. An experimental model of femoral condylar defect leading to osteoarthrosis. J OrthopTrauma. 1993;7:458–467. [DOI] [PubMed]
- 18.Lu Y, Markel MD, Swain C, Kaplan LD. Development of partial thickness articular cartilage injury in an ovine model. J Orthop Res. 2006;24:1974–1982. [DOI] [PubMed]
- 19.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hips. J Bone Joint Surg Am. 1971;53:523–537. [PubMed]
- 20.Marijnissen AC, van Roermund PM, TeKoppele JM, Bijlsma JW, Lafeber FP. The canine ‘groove’ model, compared with the ACLT model of osteoarthritis. Osteoarthritis Cartilage. 2002;10:145–155. [DOI] [PubMed]
- 21.Marijnissen AC, van Roermund PM, Verzijl N, Tekoppele JM, Bijlsma JW, Lafeber FP. Steady progression of osteoarthritic features in the canine groove model. Osteoarthritis Cartilage. 2002;10:282–289. [DOI] [PubMed]
- 22.Mitchell N, Shepard N. The deleterious effects of drying on articular cartilage. J Bone Joint Surg Am. 1989;71:89–95. [PubMed]
- 23.Mizuta H, Kudo S, Nakamura E, Takagi K, Hiraki Y. Expression of the PTH/PTHrP receptor in chondrogenic cells during the repair of full-thickness defects of articular cartilage. Osteoarthritis Cartilage. 2006;14:944–952. [DOI] [PubMed]
- 24.Pelletier JP, Martel-Pelletier J, Howell DS, Ghandur-Mnaymneh L, Enis JE, Woessner JF Jr. Collagenase and collagenolytic activity in human osteoarthritic cartilage. Arthritis Rheum. 1983;26:63–68. [DOI] [PubMed]
- 25.Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85(suppl 2):17–24. [DOI] [PubMed]
- 26.Sachs L. Applied Statistics: A Handbook of Techniques. 2nd ed. New York: Springer-Verlag; 1982.
- 27.Saris DB, Dhert WJ, Verbout AJ. Joint homeostasis. The discrepancy between old and fresh defects in cartilage repair. J Bone Joint Surg Br. 2003;85:1067–1076. [DOI] [PubMed]
- 28.Shao X, Goh JC, Hutmacher DW, Lee EH, Zigang G. Repair of large articular osteochondral defects using hybrid scaffolds and bone marrow-derived mesenchymal stem cells in a rabbit model. Tissue Eng. 2006;12:1539–1551. [DOI] [PubMed]
- 29.Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75:532–553. [DOI] [PubMed]
- 30.Speer KP, Callaghan JJ, Seaber AV, Tucker JA. The effects of exposure of articular cartilage to air: a histochemical and ultrastructural investigation. J Bone Joint Surg Am. 1990;72:1442–1450. [PubMed]
- 31.Visco DM, Hill MA, Widmer WR, Johnstone B, Myers SL. Experimental osteoarthritis in dogs: a comparison of the Pond-Nuki and medial arthrotomy methods. Osteoarthritis Cartilage. 1996;4:9–22. [DOI] [PubMed]
- 32.Walker EA. Cellular responses of embryonic hyaline cartilage to experimental wounding in vitro. J Orthop Res. 2000;18:25–34. [DOI] [PubMed]