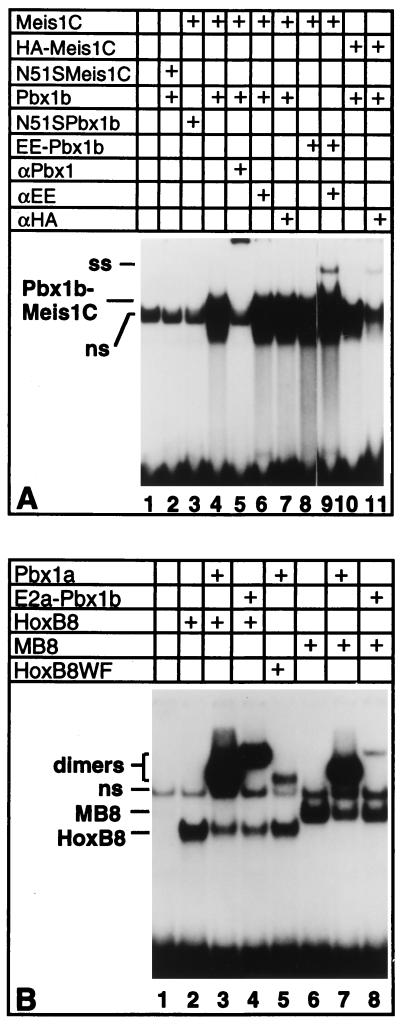
Figure 3.
Pbx1 and Meis1c are positioned on DNA in the same order as Pbx and Hox proteins. (A) DNA-binding mutants, designated N51S Meis and N51S Pbx1, and eptitope-tagged versions of Pbx1 and Meis1 were tested for complex formation potential and their ability to be supershifted or disrupted by anti-epitope antibodies. Supershift analysis was performed by addition of 200 ng of αEE or αHA mAbs. (B) Conversion of the DNA-binding specificity of Meis1 into that of HoxB8 alters its dimerization specificity with Pbx1 at the 3′ half-site. Equimolar amounts of wild-type and mutant proteins were used. Additions to the gel-shift reactions are indicated above each lane by plus signs.