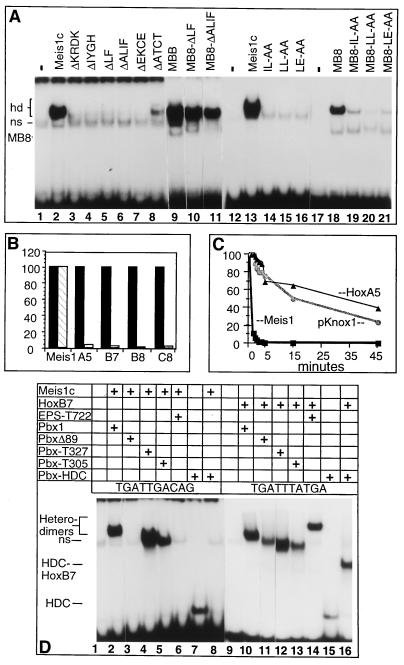
Figure 4.
Comparative properties of Pbx1–Meis1 and Pbx1–Hox complexes. (A) Wild-type and mutant forms of Meis1c and MB8 were tested for their ability to form cooperative heterodimers with Pbx1 on a TGATTGAC probe by using EMSA. Proteins added to the binding reactions are indicated at the top of each lane and correspond to the mutants depicted in Fig. 1E. (B) Disruption of Pbx1–Hox and Pbx1–Meis1c complexes by inclusion of a synthetic peptide containing the pentapeptide motif of HoxA5 was quantitated by EMSA. Filled and open bars represent complex abundance in reactions without and with peptide, respectively. (C) Off-rate analysis was performed by using cold double-stranded competitors (TGATTGAC for Pbx1–Meis1 and pKnox1 and TGATTTAT for Pbx1–HoxB7). Squares, circles, and triangles represent dissociation rates of Pbx1–Meis1, Pbx1–pKnox1, and Pbx1–HoxA5, respectively. (D) Complex formation by wild-type and mutant forms of Pbx1 with Meis1 and HoxB7 on TGATTGAC (lanes 1–8) or TGATTTAT elements (lanes 9–16), respectively, by using EMSA. Additions to the gel-shift reactions are indicated above each lane by plus signs and described in the text.